Fig. 4. AKAP95 undergoes phase separation with liquid-like properties in vitro.
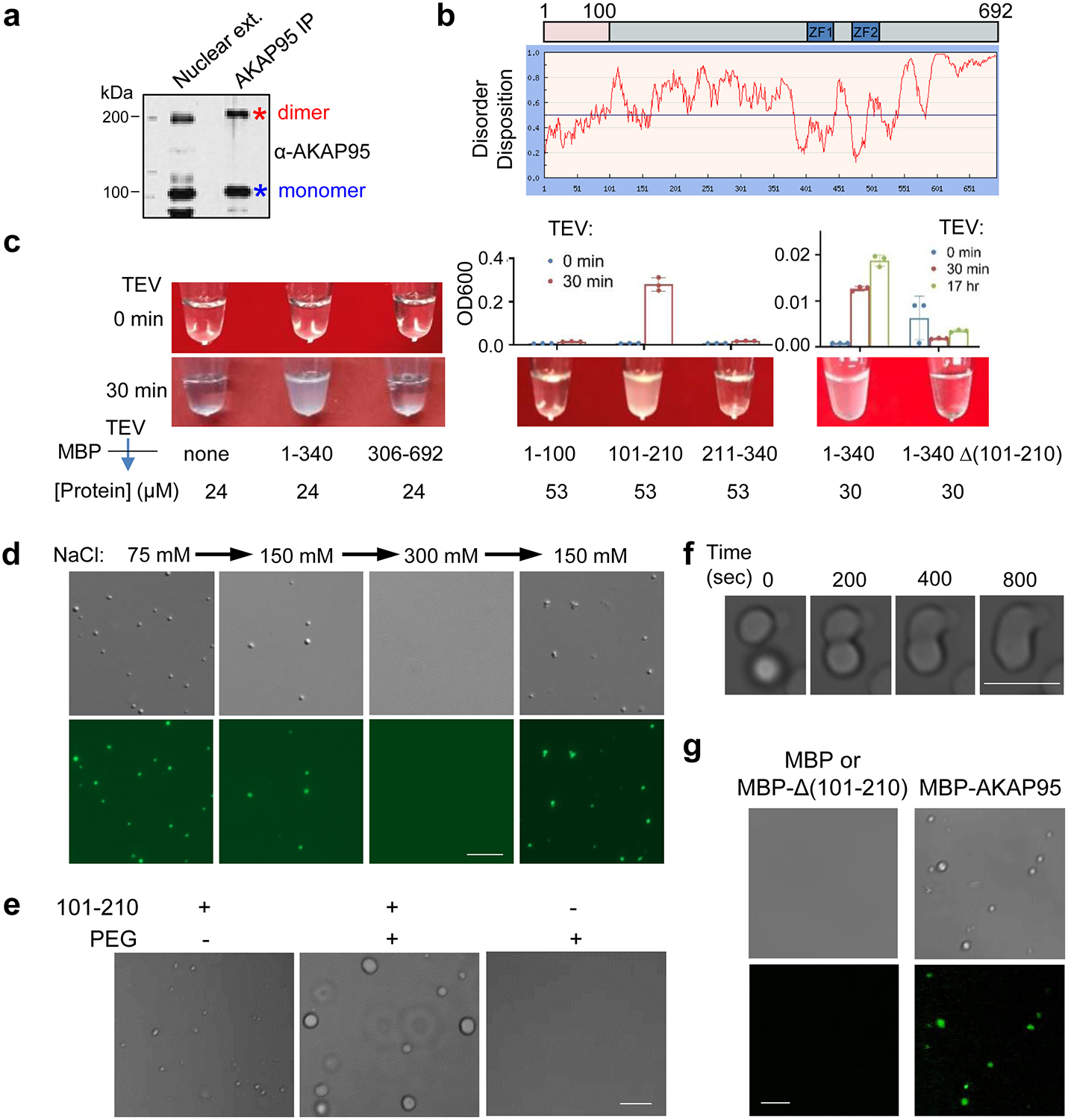
a, Immunoblotting for AKAP95 in HeLa cell nuclear extract and AKAP95 immunoprecipitation from the extract. Samples were boiled in the presence of DTT and resolved by SDS-PAGE.
b, Disorder plot of human AKAP95.
c, Turbidity by pictures and OD600 of MBP (none) and MBP fused to AKAP95 truncations at indicated concentrations all in 30 mM NaCl before and after TEV protease treatment for indicated time. OD600 is plotted as mean ± SD from n = 3 biological repeats.
d, DIC (top) and fluorescence microscopy (bottom) images for 20 μM MBP-AKAP95 (101–210) and spiked with Oregon-green-labeled same protein (molar ratio 10:1) after TEV protease treatment for 30 min. Changes in NaCl concentration is indicated. Images were taken 5 min after salt adjustment.
e, Phase contrast images of 50 μM MBP-AKAP95 (101–210) in 30 mM NaCl in the absence and presence of 10% of PEG6000 after TEV protease treatment for 30 min.
f, Fusion of two droplets formed by 50 μM MBP-AKAP95 (101–210) in 30 mM NaCl and 10% of PEG6000 after TEV protease treatment for 30 min. Also see Video 1.
g, DIC and fluorescence microscopy images of 6.25 μM MBP, MBP fused to Δ(101–210) or full-length AKAP95 in 150 mM NaCl, spiked with Oregon-green-labeled AKAP95 (101–210) at a molar ratio of 150:1 after TEV protease treatment for 30 min. Note that the lack of any condensates in the DIC images showed the inability of Δ(101–210) in condensation. Experiments in a, d, e-g were repeated 4 times.
Scale bar, 5 μm for all. Uncropped blots and statistical source data are provided as in source data fig 4.
