Abstract
The Capnodiales, which includes fungi known as the sooty moulds, represents the second largest order in Dothideomycetes, encompassing morphologically and ecologically diverse fungi with different lifestyles and modes of nutrition. They include saprobes, plant and human pathogens, mycoparasites, rock-inhabiting fungi (RIF), lichenised, epi-, ecto- and endophytes. The aim of this study was to elucidate the lifestyles and evolutionary patterns of the Capnodiales as well as to reconsider their phylogeny by including numerous new collections of sooty moulds, and using four nuclear loci, LSU, ITS, TEF-1α and RPB2. Based on the phylogenetic results, combined with morphology and ecology, Capnodiales s. lat. is shown to be polyphyletic, representing seven different orders. The sooty moulds are restricted to Capnodiales s. str., while Mycosphaerellales is resurrected, and five new orders including Cladosporiales, Comminutisporales, Neophaeothecales, Phaeothecales and Racodiales are introduced. Four families, three genera, 21 species and five combinations are introduced as new. Furthermore, ancestral reconstruction analysis revealed that the saprobic lifestyle is a primitive state in Capnodiales s. lat., and that several transitions have occurred to evolve lichenised, plant and human parasitic, ectophytic (sooty blotch and flyspeck) and more recently epiphytic (sooty mould) lifestyles.
Key words: Capnodiales, Cladosporium, Mycosphaerella, Multigene phylogeny, Sooty moulds
Taxonomic novelties: New orders: Cladosporiales Abdollahz. & Crous, Comminutisporales Abdollahz. & Crous, Neophaeothecales Abdollahz. & Crous, Phaeothecales Abdollahz. & Crous, Racodiales Abdollahz. & Crous
New families: Comminutisporaceae Abdollahz. & Crous, Neoantennariellaceae Abdollahz. & Crous, Neophaeothecaceae Abdollahz. & Crous, Readerielliopsidaceae Abdollahz. & Crous
New genera: Neoantennariella Abdollahz. & Crous, Neoasbolisia Abdollahz. & Crous, Neophaeotheca Abdollahz. & Crous
New species: Capnodium alfenasii Abdollahz. & Crous, Capnodium blackwelliae Abdollahz. & Crous, Capnodium gamsii Abdollahz. & Crous, Capnodium neocoffeicola Abdollahz. & Crous, Capnodium paracoffeicola Abdollahz. & Crous, Chaetocapnodium summerellii Abdollahz. & Crous, Chaetocapnodium indonesiacum Abdollahz. & Crous, Chaetocapnodium insulare Abdollahz. & Crous, Chaetocapnodium tanzanicum Abdollahz. & Crous, Chaetocapnodium thailandense Abdollahz. & Crous, Leptoxyphium citri Abdollahz. & Crous, Neoantennariella phylicae Abdollahz. & Crous, Neoasbolisia phylicae Abdollahz. & Crous, Phaeoxyphiella australiana Abdollahz. & Crous, Phaeoxyphiella phylicae Abdollahz. & Crous, Scolecoxyphium blechni Abdollahz. & Crous, Scolecoxyphium blechnicola Abdollahz. & Crous, Scolecoxyphium leucadendri Abdollahz. & Crous, Scolecoxyphium phylicae Abdollahz. & Crous, Scorias aphidis Abdollahz. & Crous, Scorias camelliae Abdollahz. & Crous
New combinations: Chaetocapnodium philippinense (Hongsanan & K.D. Hyde) Abdollahz. & Crous, Chaetocapnodium placitae (Cheewangkoon & Crous) Abdollahz. & Crous, Neophaeotheca salicorniae (Crous & Roets) Abdollahz. & Crous, Neophaeotheca triangularis (de Hoog & Beguin) Abdollahz. & Crous, Phragmocapnias plumeriae (Hongsanan & K.D. Hyde) Abdollahz. & Crous
Introduction
The Dothideomycetes represents a class of ecologically diverse and cosmopolitan fungi from aquatic to terrestrial ecosystems. Diverse lifestyles are found amongst the Dothideomycetes including epiphytes, endophytes, saprobes, plant and animal pathogens, mycoparasites, mycorrhizal, lichenised and rock-inhabiting fungi (Schoch et al., 2009, Schoch and Grube, 2015, Ametrano et al., 2019). The Dothideomycetes is divided into two subclasses, Pleosporomycetidae and Dothideomycetidae, and some incertae sedis lineages, accommodating more than 25 orders, 110 families and over 19 000 species, thereby representing the largest class of Ascomycota (Schoch et al., 2009, Hyde et al., 2013, Jaklitsch et al., 2015, Schoch and Grube, 2015, Van Nieuwenhuijzen et al., 2016, Bezerra et al., 2017, Videira et al., 2017, Wijayawardene et al., 2017). Morphologically they are mostly characterised by ascostromatic development and bitunicate asci with fissitunicate dehiscence (Schoch & Grube 2015).
The Capnodiales represent the second largest order in Dothideomycetes after the Pleosporales. The Capnodiales is included in the subclass Dothideomycetidae along with the Dothideales and Myrangiales (Crous et al. 2009). The taxonomic concept of this order was expanded from the original description by Luttrell (1955), based on a multigene phylogeny and the presence of ostiolar periphyses as a synapomorphic feature (Schoch et al. 2006). Taxa in this order lack pseudoparaphyses, but include several species with periphysoids and periphyses (Lumbsch & Lindemuth 2001).
As discussed by Schoch & Grube (2015), the Capnodiales was established based on the sooty moulds in three families, Antennulariaceae, Capnodiaceae, and Coccodiniaceae. However, phylogenetic analyses revealed that the sooty moulds are polyphyletic and include species residing in two different classes, Dothideomycetes and Eurotiomycetes (Crous et al. 2007a).
The Capnodiales now includes the epiphytic sooty moulds associated with honeydew produced by insects (Antennulariellaceae, Capnodiaceae, Euantennariaceae, Metacapnodiaceae), hyperparasites, rock-inhabiting fungi, ectophytes, saprobes, endophytes and pathogens associated with plants and humans (Cladosporiaceae, Cystocoleaceae, Dissoconiaceae, Extremaceae, Mycosphaerellaceae, Neodevriesiaceae, Schizothyriaceae, Phaeothecaceae (including Phaeotheca fissurella and Phaeotheca shathenatiana), Phaeothecoidiellaceae, Teratosphaeriaceae (including Piedraiaceae), Comminutispora, Phaeotheca (P. salicorniae and P. triangularis) and lichenised species (Cystocoleaceae and Racodium) (Hughes, 1976, Aptroot, 2006, Crous et al., 2007a, Crous et al., 2009, Crous et al., 2016, Crous et al., 2018, Quaedvlieg et al., 2014, Hongsanan et al., 2017, Lücking et al., 2017, Videira et al., 2017).
During the course of the past decade, considerable attention has been paid to the phylogeny and systematics of genera and families in the Capnodiales. Presently the order accommodates fungi having highly diverse ecological niches, lifestyles and modes of nutrition (Crous et al., 2007a, Crous et al., 2009, Ruibal et al., 2009, Schoch et al., 2009, Hyde et al., 2013, Chomnunti et al., 2014, Quaedvlieg et al., 2014, Ismail et al., 2016, Hongsanan et al., 2017, Videira et al., 2017, Crous et al., 2018). Although the Capnodiales s. str. are epiphytic sooty moulds, the presently applied circumscription also includes ectophytes and plant pathogens. Previous studies have, however, not addressed this ecological divergence adequately. This is due to a limited sampling of sooty moulds, and a poorly resolved phylogenetic backbone mainly based on nuclear ribosomal RNA genes. The aim of this study was therefore to reconsider the phylogenetic backbone of the Capnodiales by including numerous new collections of sooty moulds, thus also providing a more robust phylogeny using four nuclear loci, LSU, ITS, TEF-1α and RPB2.
Materials and methods
Isolates
The sooty mould isolates studied here were obtained from the culture collection (CBS) of the Westerdijk Fungal Biodiversity Institute (WI), Utrecht, the Netherlands, and the working collection of Pedro Crous (CPC) housed at the WI (Table 1). Sequences of other strains were retrieved from GenBank (Tables 1, S1). Representative cultures of the new species described in this study were deposited in the CBS culture collection.
Table 1.
Details of the sooty mould isolates included in this study. Type cultures and sequences generated in this study are in bold face.
| Family and Species name | Voucher/Culture1 | Substrate/Lifestyle2 | Country/Location | Collector | GenBank accession numbers3 |
||||
|---|---|---|---|---|---|---|---|---|---|
| LSU | ITS | TEF-1α | RPB2 | ||||||
| Capnodiaceae | |||||||||
| Capnodium alfenasii | CBS 146151 = CPC 22666 | Tabebuia sp. | Brazil | A.C. Alfenas | MN749165 | MN749233 | MN829346 | MN829260 | |
| CBS 146152 = CPC 22667 | Tabebuia sp. | Brazil | A.C. Alfenas | MN749166 | MN749234 | MN829347 | MN829261 | ||
| Ca. blackwelliae | CBS 133588 = CPC 14327 | Myrtus communis | USA | P.W. Crous | MH878118 | MN749235 | GU349054 | GU371743 | |
| Ca. coartatum | MFLUCC10-0069 | Psidium sp. | Thailand | P. Chomnunti | JN832614 | – | – | – | |
| MFLUCC10-0070 | – | Thailand | P. Chomnunti | JN832615 | – | – | – | ||
| CPC 17779 | Alstonia scholaris | Thailand | K.D. Hyde | MN749167 | MN749236 | MN829348 | MN829262 | ||
| Ca. coffeae | CBS 147.52 = AFTOL-ID 939 | Coffea robusta | Zaire | Deposited by J. Nicot/Isolated by A. Saccas | GU214400 | DQ491515 | DQ471089 | KT216519 | |
| Ca. coffeicola | MFLUCC15-0206 | Coffea sp. | Thailand | S. Hongsanan | KU358920 | KU358921 | – | – | |
| Ca. gamsii | CBS 892.73 | Sooty mould, on unknown leaf | Sri Lanka | W. Gams | GU301847 | MN749237 | GU349045 | GU371736 | |
| CBS 146153 = CPC 17765 | Lagerstroemia speciosa | Thailand | K.D. Hyde | MN749168 | MN749238 | MN829349 | MN829263 | ||
| MFLUCC10-0066 | – | Thailand | S.K. Chandranath | JN832613 | – | – | – | ||
| CBS 146154 = CPC 20466 = MFLUCC12-0101 | Lagerstroemia floribunda | Thailand | S. Hongsanan | MN749169 | MN749239 | MN829350 | MN829264 | ||
| CBS 146155 = CPC 20467 = MFLUCC12-0102 | Lagerstroemia floribunda | Thailand | S. Hongsanan | MN749170 | MN749240 | MN829351 | MN829265 | ||
| CBS 146156 = CPC 20471 = MFLUCC12-0107 | Living leaf of unknown host | Thailand | S. Hongsanan | MN749171 | MN749241 | MN829352 | MN829266 | ||
| Ca. neocoffeicola | CBS 139614 = MFLUCC14-0570 | Coffea arabica | Thailand | S. Hongsanan | MN749172 | MN749242 | MN829353 | MN829267 | |
| CBS 139613 = MFLUCC14-0569 | Coffea arabica | Thailand | S. Hongsanan | MN749173 | MN749243 | MN829354 | MN829268 | ||
| Ca. paracoffeicola | CBS 139616 = MFLUCC 14-0572 | Coffea arabica | Thailand | S. Hongsanan | MN749174 | MN749244 | MN829355 | MN829269 | |
| CBS 139615 = MFLUCC14-0571 | Coffea arabica | Thailand | S. Hongsanan | MN749175 | MN749245 | MN829356 | MN829270 | ||
| Chaetocapnodium indonesiacum | CBS 202.30 | Camelia sinensis | Indonesia | Deposited by F.H. van Beyma/Isolated by Steinmann | GU301849 | MH855113 | GU349060 | MN829273 | |
| Ch. insulare | CBS 146159 = CPC 19221 | Phylica arborea | South Africa | M.J. Wingfield | MN749178 | MN749248 | MN829359 | MN829274 | |
| CBS 146160 = CPC 19223 | Phylica arborea | South Africa | M.J. Wingfield | MN749179 | MN749249 | MN829360 | MN829275 | ||
| CBS 146161 = CPC 19224 | Phylica arborea | South Africa | M.J. Wingfield | MN749180 | MN749250 | MN829361 | MN829276 | ||
| Ch. philippinense | MFLUCC12-0110 = CPC 20474 | Palm | Philippines | K.D. Hyde | KP744503 | MN749251 | MN829362 | MN829277 | |
| Ch. placitae | CBS 124758 = CPC 13706 | Eucalyptus placita | Australia | B.A. Summerell | GQ303299 | GQ303268 | MN829363 | MN829278 | |
| Ch. siamensis | MFLUCC13-0778 | Leaves of unidentified plant | Thailand | S. Hongsanan | KP744479 | – | – | – | |
| CBS 139815 = MFLUCC13-0096 | Leaves of unidentified plant | Thailand | S.C. Karunarathna | MN749181 | MN749252 | MN829364 | MN829279 | ||
| Ch. summerellii | CBS 146157 = CPC 13654 | Eucalyptus placita | Australia | B.A. Summerell | MN749176 | MN749246 | MN829357 | MN829271 | |
| CBS 146158 = CPC 17368 | – | Laos | P. Pheng | MN749177 | MN749247 | MN829358 | MN829272 | ||
| Ch. tanzanicum | CBS 145.79 | Lichen | Tanzania | – | MN749182 | MN749253 | MN829365 | MN829280 | |
| Ch. thailandense | CBS 139619 = MFLUCC13-0787 | – | Thailand | S.C. Karunarathna | MN749183 | MN749254 | MN829366 | MN829281 | |
| Conidiocarpus asiticus | MFLUCC10-0062 | Coffea arabica | Thailand | J.K. Liu | JN832612 | KU358924 | – | – | |
| Co. caucasicus | GUMH 937 | Citrus sinensis | Iran | F. Byrami | KC833050 | – | – | – | |
| Co. siamensis | MFLUCC10-0064 | Mangifera indica | Thailand | R. Phokhomsak | JN832609 | – | – | – | |
| Co. siamensis | MFLUCC10-0061 | – | Thailand | P. Chomnunti | JN832607 | KU358923 | – | – | |
| Co. siamensis | MFLUCC10-0063 | Coffea arabica | Thailand | J.K. Liu | JN832608 | KU358925 | – | – | |
| Conidiocarpus sp. | CPC 17778 | Guave sp. | Thailand | K.D. Hyde | MN749185 | MN749256 | MN829368 | MN829283 | |
| CPC 20463 = MFLUCC12-0098 | Malus sp. | Thailand | W. Saowanee | MN749187 | MN749258 | MN829370 | MN829285 | ||
| CPC 20464 = MFLUCC12-0099 | Mimusops elengi | Thailand | S. Hongsanan | MN749194 | MN749265 | MN829377 | MN829292 | ||
| CPC 20465 = MFLUCC12-0100 | Mimusops elengi | Thailand | S. Hongsanan | MN749191 | MN749262 | MN829374 | MN829289 | ||
| CPC 20468 = MFLUCC12-0103 | Mango | Thailand | Puttaluk | MN749193 | MN749264 | MN829376 | MN829291 | ||
| CPC 20472 = MFLUCC12-0108 | Living leaf of unknown host | Thailand | S. Hongsanan | MN749188 | MN749259 | MN829371 | MN829286 | ||
| CPC 21380 = MFLUCC12-0404 | Malus sp. | Thailand | K.D. Hyde | MN749186 | MN749257 | MN829369 | MN829284 | ||
| CBS 139818 = MFLUCC14-0874 | Coffea arabica | Thailand | S. Hongsanan | MN749190 | MN749261 | MN829373 | MN829288 | ||
| CBS 139819 = MFLUCC14-0875 | Coffea arabica | Thailand | S. Hongsanan | MN749192 | MN749263 | MN829375 | MN829290 | ||
| CBS 139820 = MFLUCC 14-0876 | Coffea arabica | Thailand | S. Hongsanan | MN749184 | MN749255 | MN829367 | MN829282 | ||
| CBS 139821 = MFLUCC14-0877 | Coffea arabica | Thailand | S. Hongsanan | MN749189 | MN749260 | MN829372 | MN829287 | ||
| Heteroconium citharexyli | HM628775 | Citharexylum ilicifolium | Ecuador | H. Sydow | HM628775 | HM628776 | – | – | |
| Leptoxyphium cacuminum | MFLUCC10-0059 | Gossypium herbaceum | Thailand | S.C. Karunarathna | JN832603 | – | – | – | |
| MFLUCC10-0049 | Mimusops elengi | Thailand | P. Chomnunti | JN832602 | – | – | – | ||
| MFLUCC10-0086 | Ficus sp. | Thailand | K.D. Hyde | JN832604 | – | – | – | ||
| L. citri | CBS 451.66 | Citrus sinensis | Spain | H.A. van der Aa | KF902094 | MN749266 | GU349039 | GU371727 | |
| CBS 146162 = CPC 26196 | – | – | V. Guarnaccia | MN749195 | MN749267 | MN829378 | MN829294 | ||
| L. glochidion | IFRDCC 2651 | Glochidion wrightii | China | H. Yang | KF982308 | KF982307 | – | – | |
| L. kurandae | CBS 129530 = CPC 17274 | Eucalyptus sp. | Australia | P.W. Crous & R.G. Shivas | JF951170 | JF951150 | MN829379 | MN829295 | |
| L. madagascariense | CBS 124766 = CPC 14623 | Eucalyptus camaldulensis | Madagascar | M.J. Wingfield | MH874923 | MH863407 | MN829380 | MN829296 | |
| Leptoxyphium sp. | CPC 17767 | Gossypium herbaceum | Thailand | K.D. Hyde | MN749203 | MN749275 | MN829388 | MN829304 | |
| CPC 20470 = MFLUCC12-0106 | Living leaf of unknown host | Thailand | S. Hongsanan | MN749200 | MN749272 | MN829385 | MN829301 | ||
| CPC 20473 = MFLUCC12-0109 | Living leaf of unknown host | Thailand | S. Hongsanan | MN749197 | MN749269 | MN829382 | MN829298 | ||
| CPC 20481 = MFLUCC12-0118 | Living leaf of unknown host | Thailand | – | MN749201 | MN749273 | MN829386 | MN829302 | ||
| CPC 21382 = MFLUCC12-0406 | Heliconia sp. | Thailand | S. Hongsanan | MN749199 | MN749271 | MN829384 | MN829300 | ||
| CPC 21383 = MFLUCC12-0407 | Ixora chinensis | Thailand | S. Hongsanan | MN749202 | MN749274 | MN829387 | MN829303 | ||
| CBS 123.26 = ATCC 11925 = IMI 089363 | Hibiscus tiliaceus | Indonesia | Deposited by M.B. Schwarz | GU214430 | MH854862 | GU349051 | GU371741 | ||
| CBS 382.87 | Citrus aurantium | India | Deposited and isolated by N.D. Sharma | MN749205 | MN749277 | MN829390 | MN829306 | ||
| CBS 135836 | Insect gut | India | S. Kajale & M. Sonawane | MN749206 | MN749278 | MN829391 | MN829307 | ||
| CBS 139617 = MFLUCC13-0781 | – | Thailand | S. Hongsanan | MN749196 | MN749268 | MN829381 | MN829297 | ||
| CBS 139618 = MFLUCC13-0783 | – | Thailand | S. Hongsanan | MN749204 | MN749276 | MN829389 | MN829305 | ||
| CBS 139620 = MFLUCC13-0786 | – | Thailand | S.C. Karunarathna | MN749207 | MN749279 | MN829392 | MN829308 | ||
| CBS 139812 = MFLUCC13-0078 | Living leaf of unknown host | Thailand | S.C. Karunarathna | MN749208 | MN749280 | MN829393 | MN829309 | ||
| CBS 139814 = MFLUCC13-0790 | Living leaf of unknown host | Thailand | S.C. Karunarathna | MN749198 | MN749270 | MN829383 | MN829299 | ||
| Phragmocapnias betle | CPC 17762 | Mimusops elengi (Bullet wood) | Thailand | K.D. Hyde | MN749221 | MN749293 | MN829407 | MN829323 | |
| CPC 20476 = MFLUCC12-0112 | Palm | Philippines | K.D. Hyde | MN749222 | MN749294 | MN829408 | MN829324 | ||
| CPC 21379 = MFLUCC12-0403 | Malus sp. | Thailand | K.D. Hyde | MN749223 | MN749295 | MN829409 | MN829325 | ||
| MFLUCC10-0053 | Ixora sp. | Thailand | P. Chomnunti | JN832606 | KU358922 | – | – | ||
| Ph. plumeriae | MFLUCC15-0205 | Plumeria sp. | Thailand | C. Singhapop | KU358918 | KU358919 | – | – | |
| Polychaeton citri | CBS 116435 | Citrus aurantium | Iran | R. Zare & W. Gams | GU214469 | GU214649 | MN829394 | MN829310 | |
| Neoantennariellaceae | |||||||||
| Fumiglobus pieridicola | UBC F23788 | Pieris japonica | Canada | Tanay Bose | KC833052 | KF263961 | – | – | |
| Neoantennariella phylicae | CBS 146164 = CPC 19227 | Phyllica arborea | South Africa | M.J. Wingfield | MN749209 | MN749281 | MN829395 | MN829311 | |
| CBS 146165 = CPC 19977 | Phylica arborea | UK | P. Ryan | MN749213 | MN749285 | MN829399 | MN829315 | ||
| CBS 146166 = CPC 19981 | Phylica arborea | UK | P. Ryan | MN749212 | MN749284 | MN829398 | MN829314 | ||
| CBS 146167 = CPC 19985 | Phylica arborea | UK | P. Ryan | MN749210 | MN749282 | MN829396 | MN829312 | ||
| CPC 19992 | Phylica arborea | UK | P. Ryan | MN749214 | MN749286 | MN829400 | MN829316 | ||
| CBS 146163 = CPC 19989 | Phylica arborea | UK | P. Ryan | MN749211 | MN749283 | MN829397 | MN829313 | ||
| Neoasbolisia phylicae | CBS 146168 = CPC 19982 | Phylica arborea | UK | P. Ryan | MN749215 | MN749287 | MN829401 | MN829317 | |
| Readerielliopsidaceae | |||||||||
| “Capnodium” salicinum | CBS 131.34 = AFTOL-ID 937 | Bursaria spinosa | Indonesia | Deposited by E.E. Fisher | EU019269 | AJ244240 | DQ677889 | KT216553 | |
| Phaeoxyphiella australiana | CBS 146169 = CPC 29527 | Agonis sp. | Australia | P.W. Crous | MN749220 | MN749292 | MN829406 | MN829322 | |
| Ph. phylicae | CBS 146171 = CPC 19979 | Phylica arborea | UK | P. Ryan | MN749216 | MN749288 | MN829402 | MN829318 | |
| CBS 146172 = CPC 19984 | Phylica arborea | UK | P. Ryan | MN749217 | MN749289 | MN829403 | MN829319 | ||
| CBS 146173 = CPC 19987 | Phylica arborea | UK | P. Ryan | MN749218 | MN749290 | MN829404 | MN829320 | ||
| CBS 146170 = CPC 19993 | Phylica arborea | UK | P. Ryan | MN749219 | MN749291 | MN829405 | MN829321 | ||
| Readerielliopsis fuscoporiae | CBS 139900 = CPC 24637 | Fuscoporia wahlbergii | French Guiana | C. Decock | KR476755 | KR476720 | MN829410 | MN829326 | |
| R. guyanensis | CBS 117550 = MUCL 46082 | Dead, decaying leaf, unidentified angiosperm in leaf litter | French Guiana | Deposited by C. Decock/Isolated by M.H. de Frahan | FJ493211 | MH863023 | MN829411 | MN829327 | |
| Scolecoxyphium blechni | CBS 146174 = CPC 19990 | Blechnum palmiforme | UK | P. Ryan | MN749224 | MN749296 | MN829412 | MN829328 | |
| Sc. blechnicola | CBS 146175 = CPC 19991 | Blechnum palmiforme | UK | P. Ryan | MN749225 | MN749297 | MN829413 | MN829329 | |
| Sc. leucadendri | CBS 146176 = CPC 18313 | Leucadendron sp. | South Africa | P.W. Crous | MN749226 | MN749298 | MN829414 | MN829330 | |
| Sc. phylicae | CBS 146177 = CPC 19219 | Phyllica arborea | South Africa | M.J. Wingfield | MN749227 | MN749299 | MN829415 | MN829331 | |
| CBS 146178 = CPC 19225 | Phyllica arborea | South Africa | M.J. Wingfield | MN749228 | MN749300 | MN829416 | MN829332 | ||
| Scorias aphidis | CBS 325.33 | Aphid | – | Deposited by L.H. Leonian | MH866910 | GU214696 | MN829417 | KT216542 | |
| Sc. camelliae | CBS 201.30 | Camellia sinensis | Indonesia | Deposited by F.H. van Beyma/Isolated by Steinmann | MH866560 | MH855112 | MN829418 | MN829333 | |
| Sc. leucadendri | CBS 131318 = CPC 18312 | Laucadendron muirii | South Africa | P.W. Crous | JQ044456 | JQ044437 | MN829419 | MN829334 | |
| CPC 17088 | Callistemon sp. | Australia | P.W. Crous | MN749229 | MN749301 | MN829420 | MN829335 | ||
| Sc. mangiferae | MFLUCC15-0230 | Mangifera indica | Thailand | S. Hongsanan | KT588603 | KT588604 | – | – | |
| Sc. spongiosa | MFLUCC10-0084 | Entada sp. | Thailand | P. Chomnunti | JN832601 | – | – | – | |
| Outgroup | |||||||||
| Elsinoe phaseoli | CBS 165.31 = AFTOL-ID 1855 = IMI 303278 | Paseolus lunatus | Cuba | Deposited by A.E. Jenkins/Isolated by C. Aguiar | DQ678095 | KX887263 | DQ677935 | KX887144 | |
| Myriangium hispanicum | CBS 247.33 | Acer monspessulanum | – | Deposited by J.B. Martínez/Isolated by H. Diddens | GU301854 | KX887304 | GU349055 | GU371744 | |
ATCC: American Type Culture Collection, Virginia, USA; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; CPC: Culture collection of Pedro Crous, housed at Westerdijk Fungal Biodiversity Institute; IFRDCC: International Fungal Research & Development Centre Culture Collection, Chinese Academy of Forestry, Kunming, China; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, United Kingdom; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Ria, Thailand; MUCL: Université Catholique de Louvain, Louvain-la-Neuve, Belgium.
Lifestyle of all sooty mould strains coded as epiphyte.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: partial 28S large subunit RNA gene; TEF-1α: partial translation elongation factor 1-alpha gene; RPB2: partial RNA polymerase II second largest subunit gene. Bold GenBank accession numbers for sequences generated in this study; – indicates unavailable sequence.
DNA extraction, PCR amplification and sequencing
Total genomic DNA was extracted from fresh mycelia grown on malt extract agar (MEA) using the Wizard® Genomic DNA Purification Kit (Promega Corporation, Fitchburg, Wisconsin, USA) following the manufacturer's protocols. The D1/D2 variable domains of the 28S nrDNA (LSU) and the ITS1, 5.8 and ITS2 region of ribosomal DNA and part of RNA polymerase II second largest subunit (RPB2) and the translation elongation factor 1-alpha (TEF-1α) were amplified and sequenced using the following primer pairs: LR0R/LR5 for LSU (Vilgalys & Hester 1990), ITS5/ITS4 for ITS (White et al. 1990), fRPB2-5F/fRPB2-7cR for RPB2 (Liu et al. 1999), EF1-983F/EF1-2218R for TEF-1α (Rehner & Buckley 2005). The PCR amplifications were performed in a total volume of 12.5 μL containing 1 μL genomic DNA, 1 × NH4 reaction buffer (Bioline, Luckenwalde, Germany), 0.2 μM of each primer, 200 μM dNTPs, 3 mM MgCl2, and 0.5 U Taq DNA polymerase (Bioline). To improve amplification of RPB2 in some difficult DNA templates 4 % Bovine Serum Albumin (BSA, New England BioLabs, #B9000S) was added to the reaction mixture.
PCR conditions for LSU, ITS and TEF-1α were: an initial denaturation step of 5 min at 95 °C followed by 35 cycles of 30 s at 95 °C, 45 s at 52 °C (ITS, LSU) or 55 °C (TEF-1α) and 1 min at 72 °C, and a final elongation step of 7 min at 72 °C. Touchdown PCR was performed for amplification of RPB2 as follows: an initial denaturation at 95 °C for 5 min followed by 35 cycles of 30 s at 95 °C, 30 s at 60 °C (5–10 cycles)/56 °C (5–10 cycles)/52 °C (15–25 cycles) and 1 min at 72 °C, and a final elongation step of 7 min at 72 °C.
The PCR products were sequenced with both forward and reverse primers using an Applied Biosystems 3730xl DNA Analyzer (Thermo Fisher Scientific). The DNASTAR Lasergene SeqMan Pro v. 8.1.3. software was used to obtain consensus sequences. All new sequences were submitted to GenBank (Tables 1, S1).
Phylogenetic analyses
Generated sequences were aligned with sequences retrieved from GenBank (http://www.ncbi.nlm.nih.gov) using the online interface of MAFFT v. 7 (http://mafft.cbrc.jp/alignment/server/index.html), and manually edited in MEGA v. 7.0.21. Maximum Likelihood (ML) and Bayesian analysis (BA) were implemented for phylogenetic inferences of both single locus and concatenated alignments on the CIPRES Science Gateway portal (https://www.phylo.org/; Miller et al. 2012) using RAxML-HPC BlackBox v. 8.2.10 (Stamatakis 2014) and MrBayes v. 3.2.6 (Huelsenbeck and Ronquist, 2001, Ronquist and Huelsenbeck, 2003), respectively. The ML analyses were performed using a GTR+GAMMA substitution model and four rate classes with 1 000 bootstrap iterations. For the Bayesian analyses the optimal nucleotide substitution models were determined for each locus using MrModelTest v. 2.3 (Nylander 2004). Bayesian analyses were computed under the optimal nucleotide substitution models with four simultaneous Markov Chain Monte Carlo chains, 10 M generations and a sampling frequency of 1 000 generations, ending the run automatically when standard deviation of split frequencies dropped below 0.01. Burn-in was set to remove 25 % of the first sampled trees, after which the 50 % majority rule consensus trees and posterior probability (PP) values were calculated. The resulting trees were plotted using FigTree v. 1.4.3 (http://tree.bio.ed.ac.uk/software/figtree). Alignments and trees were deposited in TreeBASE (www.treebase.org; S25414) and taxonomic novelties in MycoBank (www.MycoBank.org; Crous et al. 2004).
Ancestral states were reconstructed using Mesquite v.3.6 (Maddison & Maddison 2018). Character history was inferred using the Bayesian tree (see above) as phylogenetic framework. Ancestral states were determined based on a maximum likelihood approach with a MK1 model of evolution. Characters states were defined as saprobe, epiphyte, parasite or lichen. The character state for taxa with an uncertain lifestyle was coded as “?”.
Morphology
Isolates stored in liquid nitrogen or lyophilised were reactivated on 2 % malt extract agar (MEA) or oatmeal agar (OA). Colonies were sub-cultured onto MEA, OA, cornmeal agar (CMA), potato dextrose agar (PDA), and synthetic nutrient-poor agar (SNA) supplemented with pine needles at room temperature. Culture media were prepared as described by Crous et al., 2019a, Crous et al., 2019b. Cultures were examined periodically for the development of reproductive structures. Slide preparations were made with clear lactic acid or Shear's mounting fluid. Morphological observations of fungal structures were made using a Nikon SMZ1000 dissecting microscope and a Zeiss Axioscope 2 compound microscope with differential interference contrast (DIC) illumination. Measurements and images were taken using a Nikon DS-Ri2 high definition colour digital camera. Measurements and descriptions of microscopic structures were made from cultures grown on SNA. A few strains that were sterile on SNA were described from other media (indicated in text). The mean, standard deviation, maximum and minimum values of at least 30 fungal structures were calculated where possible. Dimensions are presented as a range with extremes in parentheses. Growth rates were measured on MEA after 2 wk and colony characters were noted. Colony colours were rated according to the colour chart of Rayner (1970).
Results
Phylogeny
Amplification of the partial sequences of LSU, ITS and TEF-1α was successful but RPB2 proved difficult to amplify using normal PCR, and therefore a touchdown PCR program was used. For most of the isolates the combination of fRPB2-f5F and fRPB2-7cR primers (Liu et al. 1999) was more successful than the primer combination fRPB2-f5F2 and fRPB2-7cR (Sung et al. 2007). For a few isolates, we used the forward primer fRPB2-f5F2 instead of fRPB2-f5F.
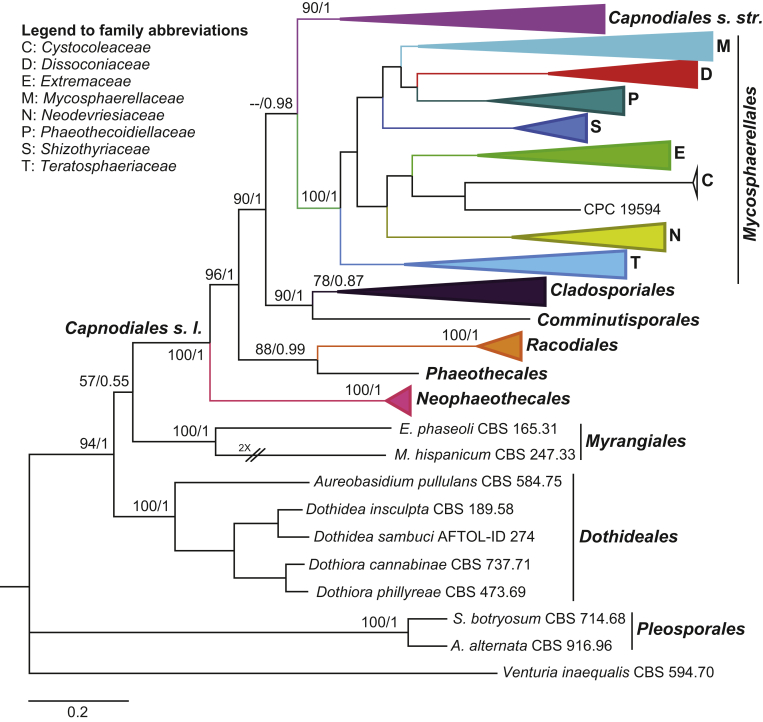
Two datasets were analysed in this study. The first dataset consisted of combined LSU, TEF-1α and RPB2, including 193 taxa representing three orders Capnodiales s. lat., Myrangiales, Dothideales, with Venturia inaequalis (CBS 594.70) as outgroup. After alignment the dataset contained a total of 3 168 characters (LSU: 837, TEF-1α: 1 176, RPB2: 1 147), including alignment gaps. MrModelTest revealed that the general time-reversible model of evolution (Rodríguez et al. 1990), including estimation of invariable sites and assuming a discrete gamma distribution (GTR+I+G) with six rate categories (lsetnst = 6, rates = invgamma) and dirichlet (1,1,1,1) base frequencies is the best nucleotide substitution model for all loci (LSU, TEF-1α and RPB2). The Bayesian analyses of the concatenated alignments of three loci generated 7 292 trees from which 1 822 trees were discarded as burn-in. The consensus tree and posterior probability values (PP) were calculated from the remaining 5 470 trees. The average standard deviation of split frequencies was 0.009987 at the end of the run. The RAxML search of the dataset with 1 767 distinct alignment patterns produced a best-scoring ML tree (lnL = -66287.001595). The bootstrap values equal to or higher than 50 % were mapped on the Bayesian tree (Figs 1, S1). The same phylogenetic tree was obtained from both RAxML and Bayesian analyses. Capnodiales s. lat. was split into seven distinct clades representing seven orders. Sooty mould fungi constituted Capnodiales s. str., a single highly supported clade (ML-BS = 90 %, PP = 1). Mycosphaerellales with high support in both analyses (ML-BS = 100 %, PP = 1) proved clearly distinct from Capnodiales s. str., and was thus resurrected here as a separate order containing eight families: Mycosphaerellaceae, Dissoconiaceae, Phaeothecoidiellaceae, Schizothyriaceae, Extremaceae, Cystocoleaceae, Neodevriesiaceae and Teratosphaeriaceae (Figs 1, S1). In the Bayesian analysis Mycosphaerellales grouped with Capnodiales s. str. in a well-supported clade (PP = 0.98), while in the RAxML analysis it was a sister group of Cladosporiaceae with low support (ML-BS < 50 %). Therefore, Cladosporiaceae was elevated to ordinal level, and Cladosporiales introduced. In both RAxML and Bayesian analyses four new orders were recognised: Phaeothecales, Neophaeothecales, Racodiales (for Racodium rupestre), and Comminutisporales (for Comminutispora agavaciensis).
Fig. 1.
Reduced phylogenetic tree inferred from a Bayesian analysis based on a concatenated alignment of LSU, TEF-1α and RPB2. Bayesian posterior probabilities (PP) and maximum likelihood bootstrap support values (ML-BS) are indicated at the nodes (PP/ML-BS). The scale bar represents the expected number of changes per site. The lineages in Capnodiales s. l. are indicated in different colours. The tree was rooted with Venturia inaequalis (CBS 594.70).
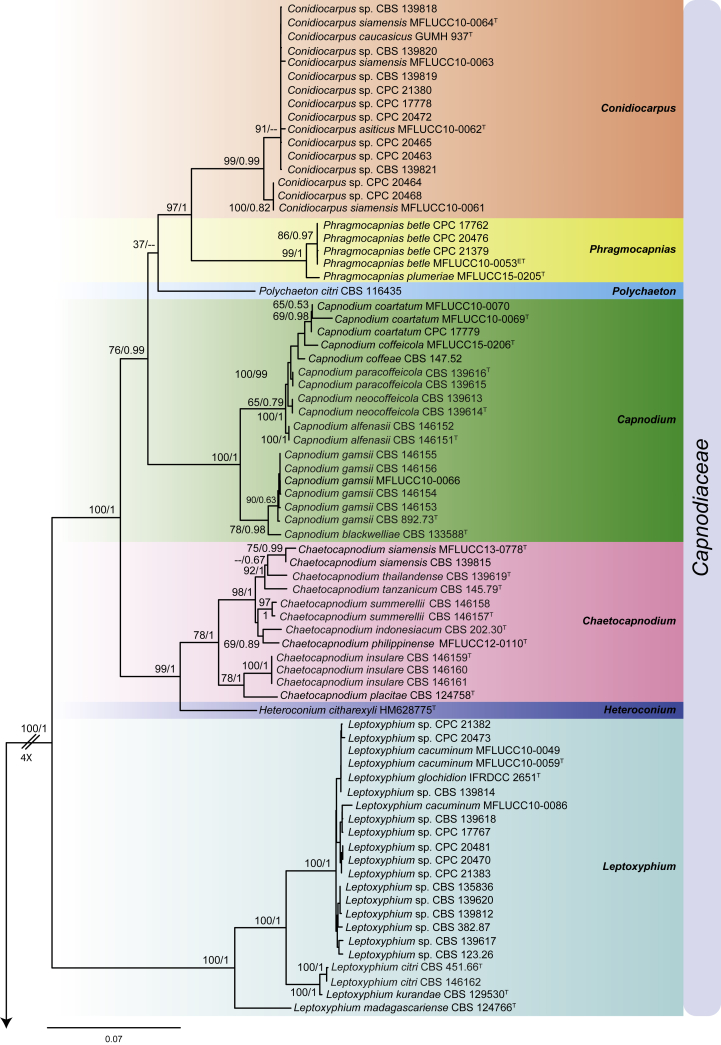
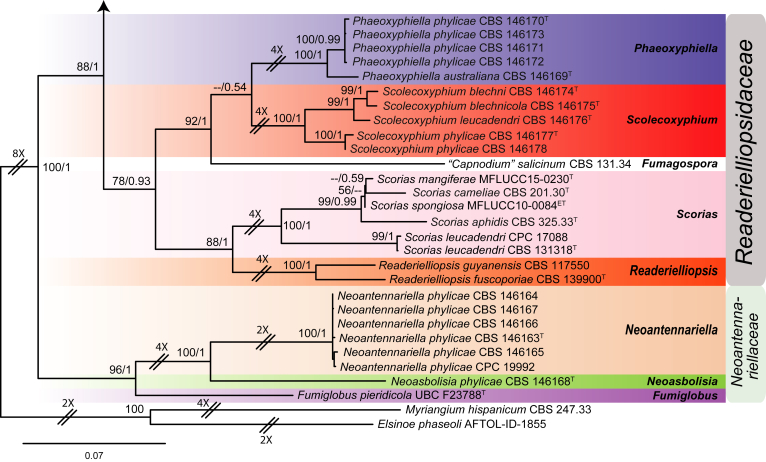
The second dataset consisted of aligned sequences of four loci (LSU, ITS, TEF-1α and RPB2), and included 102 taxa belonging to Capnodiales s. str., and two species, Myriangium hispanicum (CBS 247.33) and Elsinoe phaseoli (AFTOL-ID-1855), as the outgroup taxa. The aligned dataset contained 3 603 characters (LSU: 849, ITS: 553, TEF-1α: 1 035, RPB2: 1 154), including alignment gaps. Results from MrModelTest indicated a GTR+I+G as the best fit model for the ITS sequence data, as was the case for three other loci (LSU, TEF-1α and RPB2) in dataset 1. The RAxML search of the second dataset detected 1 463 distinct alignment patterns and yielded a tree with lnL = -30052.650187 (Fig. 2). The Bayesian analyses generated 4 622 trees from which 1 154 trees were discarded as burn-in. The consensus tree and posterior probability values (PP) were calculated from the remaining 3 468 trees. The average standard deviation of split frequencies was 0.009980 at the end of the run. Posterior probability values were mapped on the ML tree (Fig. 2). Three families, namely Capnodiaceae, Neoantennariellaceae and Readerielliopsidaceae were recognised in Capnodiales s. str. In Capnodiaceae seven morphologically and phylogenetically well-supported genera including Capnodium, Chaetocapnodium, Conidiocarpus, Heteroconium, Leptoxyphium, Phragmocapnias and Polychaeton were identified. Eleven new species were recognised in this family. Readerielliopsidaceae contained four genera (Phaeoxyphiella, Readerielliopsis, Scolecoxyphium and Scorias) and Neoantennariellaceae three genera (Fumiglobus, Neoantennariella and Neoasbolisia).
Fig. 2.
Phylogenetic tree inferred from a RAxML search of a concatenated alignment of LSU, ITS, TEF-1α and RPB2. Maximum likelihood bootstrap support values (ML-BS) and Bayesian posterior probabilities (PP) are indicated at the nodes (ML-BS/PP). The scale bar represents the expected number of changes per site. Families and orders are highlighted in blocks of different colour and indicated to the right of the tree. The tree was rooted with Myriangium hispanicum (CBS 247.33) and Elsinoe phaseoli (AFTOL-ID-1855). T Ex-type, ET Ex-epitype.
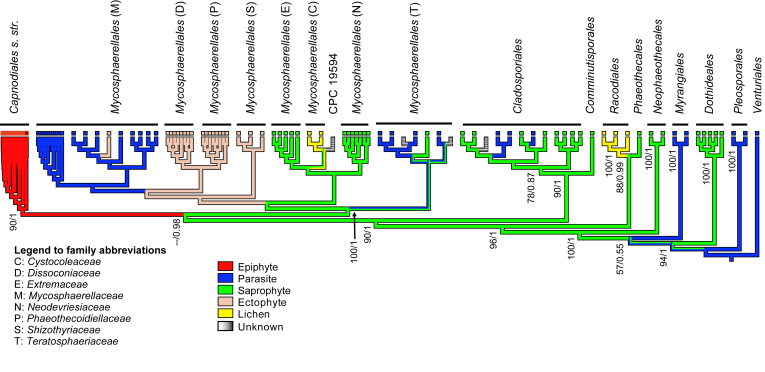
Ancestral state reconstruction revealed a saprobic lifestyle as the ancestral state of the Capnodiales s. lat. included in this study (see Neophaeothecales; Fig. 3), while whole genome sequences of a more diverse set of taxa also supported the ancestral state of Dothideomycetes to be saprobic (Haridas et al. 2020). In the dataset included in the present study the saprobic lifestyle emerged during the evolution of diverse taxa, with several reversals back to parasitism. All Capnodiales emerged from an ancestor that had an ectophytic lifestyle. The analyses also revealed that all Mycosphaerellales shared an ancestor that was saprobic. From this ancestor ectophytes and lichen associated fungi emerged, while the ancestors of several species in this group reverted to a parasitic lifestyle. The ancestor of all Cladosporiales and Comminutisporales were saprobes but some species in the Cladosporiales reverted back to parasitism. Results showed that the ancestor of the Racodiales was a lichen-associated fungus, and that of the Dothideales was a saprobe.
Fig. 3.
Cladogram showing the ancestral state reconstruction and evolution of lifestyles over the tree. Maximum likelihood bootstrap support values (ML-BS) and Bayesian posterior probabilities (PP) are indicated at the nodes (ML-BS/PP).
Taxonomy
Based on the phylogenetic analyses of the two datasets generated in this study, combined with the differences in morphology and ecology, the Capnodiales requires redefinition, and the Mycosphaerellales must be resurrected. Furthermore, five new orders, four new families, three new genera, 21 new species and five new combinations are introduced below.
Capnodiales Woron. Ann. Mycol. 23: 177. 1925.
Note: Treated below as Capnodiales s. str.
Cladosporiales Abdollahz. & Crous, ord. nov. MycoBank MB833140.
Etymology: Name refers to the genus Cladosporium.
Saprobic, endophytic, fungicolous, lichenicolous, human and plant pathogen. Ascomata pseudothecial, gregarious or scattered, immersed, black to red-brown, globose to subglobose, uniloculate, with 1(−3) short, periphysate ostiolar necks. Ostiole necks periphysoid. Hamathecium of hyaline, septate, subcylindrical pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, sessile to short-stalked, obovoid to broadly ellipsoid or subcylindrical, straight to slightly curved. Ascospores bi- to multi-seriate, or overlapping, hyaline, obovoid to ellipsoid-fusiform, with irregular luminar inclusions. Asexual morphs hyphomycetous. Conidiophores macronematous, mononematous, simple or branched, brown. Conidiogenous cells integrated, terminal and intercalary, sympodial or synchronous, mostly polyblastic, conidiogenous loci conspicuous, darkened-refractive or not. Conidia mostly in branched or unbranched acropetal chains, subhyaline to brown, smooth to verrucose or echinulate, ramoconidia present or not, dry, conidium secession schizolytic (adapted from Bensch et al. 2012).
Type genus: Cladosporium Link (sexual morph Davidiella Crous & U. Braun)
Family included: Cladosporiaceae Chalm. & R.G. Archibald (based on Cladosporium).
Comminutisporales Abdollahz. & Crous, ord. nov. MycoBank MB833141.
Etymology: Name refers to the genus Comminutispora.
Saprobic. Ascomata pseudothecial, immersed, uniloculate, separate. Asci bitunicate, 8-spored. Pseudoparaphyses absent, hamathecial tissue abundant, ostiolar canal periphysate. Ascospores muriformly septate, forming secondary ascospores within the ascus. Hyphae hyaline, becoming olivaceous, forming hyaline, aseptate endoconidia (adapted from Ramaley 1996).
Comminutisporaceae Abdollahz. & Crous, fam. nov. MycoBank MB833142.
Etymology: Name refers to the genus Comminutispora.
Saprobic. Ascomata pseudothecial, immersed, uniloculate, separate. Asci bitunicate, 8-spored. Pseudoparaphyses absent, hamathecial tissue abundant, ostiolar canal periphysate. Ascospores muriformly septate, forming secondary ascospores within the ascus. Hyphae hyaline, becoming olivaceous, forming hyaline, aseptate endoconidia.
Type genus: Comminutispora A.W. Ramaley (asexual morph Hyphospora A.W. Ramaley).
Mycosphaerellales (Nannf.) P.F. Cannon, Ainsworth & Bisby's Dictionary of the Fungi Ed. 9. 2001.
Saprobic, ectophytic, lichenicolous and phytopathogenic. Ascomata immersed to semi-immersed within the pseudostroma or clypeus or superficial, solitary, globose to subglobose with protruding central ostiole, dark brown to black, scattered or clustered, gregarious. Peridium thin- to thick-walled, of several layers of textura angularis, brown to black. Hamathecium present or absent, with cellular pseudoparaphyses, anastomosing, branching, sometimes aparaphysate. Asci bitunicate, fissitunicate, 8-spored, cylindrical to cylindrical-clavate, ovoid to saccate, sessile or stipitate, apically rounded with distinct or indistinct ocular chamber. Ascospores bi-to multi-seriate, ellipsoidal to obclavate, oblong to cylindrical, hyaline to subhyaline or pale yellowish, mostly 1-septate, constricted or not, smooth or rough-walled. Asexual morphs hyphomycetous or coelomycetous (see Videira et al. 2017 for more details about asexual morphs).
Type genus: Ramularia Unger (sexual morph Mycosphaerella Johanson).
Families included: Cystocoleaceae (based on Cystocoleus), Dissoconiaceae (based on Dissoconium), Extremaceae (based on Extremus), Mycosphaerellaceae (based on Mycosphaerella), Neodevriesiaceae (based on Neodevriesia), Phaeothecoidiellaceae (based on Phaeothecoidiella), Schizothyriaceae (based on Schizothyrium, asexual morph Zygophiala), Teratosphaeriaceae (based on Teratosphaeria, asexual morph Kirramyces).
Neophaeothecales Abdollahz. & Crous, ord. nov. MycoBank MB833143.
Etymology: Name refers to the genus Neophaeotheca.
Mycelium consisting of hyaline, smooth, septate, branched hyphae, that swell in terminal or intercalary cells, developing numerous endoconidia. Endoconidia brown, verruculose, globose to obovoid, muriformly septate, bursting open to release endoconidia that are red-brown, verruculose, aseptate, ellipsoid to subglobose or irregular.
Neophaeothecaceae Abdollahz. & Crous, fam. nov. MycoBank MB833144.
Etymology: Name refers to the genus Neophaeotheca.
Mycelium consisting of hyaline, smooth, septate, branched hyphae, that swell in terminal or intercalary cells, developing numerous endoconidia. Endoconidia brown, verruculose, globose to obovoid, muriformly septate, bursting open to release endoconidia that are red-brown, verruculose, aseptate, ellipsoid to subglobose or irregular.
Neophaeotheca Abdollahz. & Crous, gen. nov. MycoBank MB833145.
Etymology: Name refers to its morphological similarity with the genus Phaeotheca.
Mycelium consisting of hyaline, smooth, septate, branched hyphae, that swell in terminal or intercalary cells, developing numerous endoconidia. Endoconidia brown, verruculose, globose to obovoid, muriformly septate, bursting open to release endoconidia that are red-brown, verruculose, aseptate, ellipsoid to subglobose or irregular.
Type species: Neophaeotheca salicorniae (Crous & Roets) Abdollahz. & Crous
Neophaeotheca salicorniae (Crous & Roets) Abdollahz. & Crous, comb. nov. MycoBank MB833146.
Basionym: Phaeotheca salicorniae Crous & Roets, Persoonia 36: 365. 2016.
Neophaeotheca triangularis (de Hoog & Beguin) Abdollahz. & Crous, comb. nov. MycoBank MB833147.
Basionym: Phaeotheca triangularis de Hoog & Beguin, Antonie van Leeuwenhoek 71: 290. 1997.
Phaeothecales Abdollahz. & Crous, ord. nov. MycoBank MB833148.
Etymology: Name refers to the genus Phaeotheca.
Mycelium consisting of hyaline to brown, smooth-walled, septate, branched hyphae, terminal or intercalary cells becoming swollen, developing numerous endoconidia. Endoconidia brown, smooth to verruculose, thin- to thick-walled, globose to obovoid, aseptate to muriformly septate (from Crous et al. 2018).
Type genus: Phaeotheca Sigler, Tsuneda & J.W. Carmich.
Family included: Phaeothecaceae (based on Phaeotheca, see Crous et al. 2018).
Racodiales Abdollahz. & Crous, ord. nov. MycoBank MB833149.
Etymology: Name refers to the genus Racodium.
Thallus filamentous, of elongated, straight hyphae, longitudinally arranged, in close association with photobiont, not corticate, dark brown to black, forming wefts or circular patches, margin not delimited; hyphae 4–7 per photobiont filament, straight and parallel, unbranched, non-nodulose. Ascomata and conidiomata not known (from Smith et al. 2009).
Type genus: Racodium Fr.
Family included: Racodiaceae (based on Racodium).
Notes: The typification of Racodium Fr. (based on R. rupestre Pers.) was discussed by Hawksworth et al. (2011). Based on the sequences included here, Racodium (Racodiaceae Link) represents an undescribed order.
Capnodiales s. str.
The genera delineated in Fig. 2 are treated alphabetically based on order.
Capnodiales Woron. Ann. Mycol. 23: 177. 1925.
Widespread in tropical and subtropical areas, occurring on honeydew excretions from insects, forming a black, sooty growth on green, healthy leaves, stems and bark. Ascomata superficial on mycelium, subglobose to globose, with or without setae, dark brown, with a central ostiole. Pseudoparaphyses absent. Asci bitunicate, saccate, with a short pedicel, lacking an ocular chamber. Ascospores multiseptate or muriform, hyaline to brown. Asexual morphs pycnidial coelomycetous or hyphomycetous (Hughes, 1976, Crous et al., 2009, Chomnunti et al., 2011).
Type genus: Capnodium Mont.
Families included: Capnodiaceae (based on Capnodium), Neoantennariellaceae (based on Neoantennariella) and Readerielliopsidaceae (based on Readerielliopsis).
Capnodiaceae Höhn. ex Theiss., Verh. Zool.-Bot. Ges. Wien 66: 363. 1916.
Growing superficially on honeydew excretions from insects, having a black, sooty-like appearance on green leaves, stems and, bark; often co-occurring with other fungicolous taxa. Mycelium superficial on host surface, black, sooty-like, consisting of septate, branched, brown hyphae. Sexual morph: Ascomata formed in mycelial mass, subglobose to globose, setae present or lacking, dark brown, with central ostiole; peridium brown, thin-walled, cells of textura angularis. Pseudoparaphyses absent. Asci 8-spored, bitunicate, saccate, short pedicellate, generally lacking an ocular chamber. Ascospores bi- to tri-seriate, multi-septate or muriform, hyaline to brown. Asexual morphs: coelomycetous Conidiomata synnematous or pycnidial, globose to pyriform, mostly elongated, with or without necks, and with or without swelling, and central ostiole. Conidia hyaline, aseptate, ellipsoid; hyphomycetous. Conidiophores superficial, erect, brown, cylindrical, septate, proliferating percurrently at apex. Conidia brown, septate, ellipsoid or subcylindrical, solitary or in chains.
Type genus: Capnodium Mont.
Capnodium Mont., Ann. Sci. Nat. Bot. 11: 233. 1849.
Saprobic on sugary exudates from insects growing on the surface of leaves, fruits, stems and other non-plant objects. Thallus a loose or dense network of pale brown, superficial hyphae or a thick pseudoparenchymatous stromata, with sexual and asexual morphs often growing together. Ascomata superficial on mycelium, brown to dark brown or black, globose to ellipsoidal, short-stalked or sessile, ostiolate at maturity, scattered or in groups, lacking setae. Peridium comprising dark brown to pale brown, thick-walled cells forming a textura angularis. Asci 8-spored, bitunicate, clavate, ovoid or saccate, aparaphysate, apedicellate. Ascospores brown, oblong or ovoid and some reniform, transversely septate with or without one or more vertical septa. Conidiomata pycnidial, slender to flask-shaped, simple or branched, occur singly or in groups, sessile or with long or short stalk, sometimes on the same base or stalk, with or without conspicuous oval or ellipsoidal part, with short to long or without conspicuous neck, sometimes with two necks, dark brown. Ostiole at apex of pycnidia, hyphae continuing upwards to the tapered neck, terminating in an ostiole which is surrounded by obtusely rounded hyphal ends. Conidia small, ellipsoid, continuous, hyaline, aseptate (adapted from Chomnunti et al. 2011).
Type species: Capnodium citri Berk. & Desm.
Notes: The taxonomic history of Capnodium was discussed by Chomnunti et al. (2011). Index Fungorum lists 140 species names in Capnodium, while MycoBank lists 168 species names (accessed March 2019). DNA sequence data are available for only two recently published species; Ca. coartatum (LSU) and Ca. coffeicola (LSU/ITS). In the present study we sequenced LSU, ITS, TEF-1α and RPB2 loci for 13 isolates. Phylogenetic analyses revealed that these isolates represent six species, five of which are described as new below.
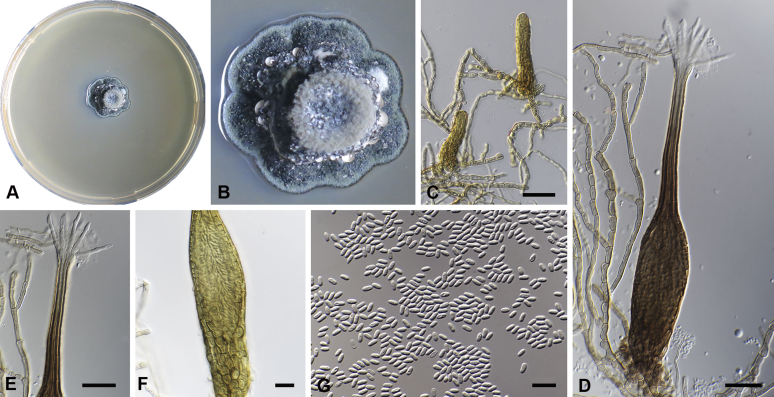
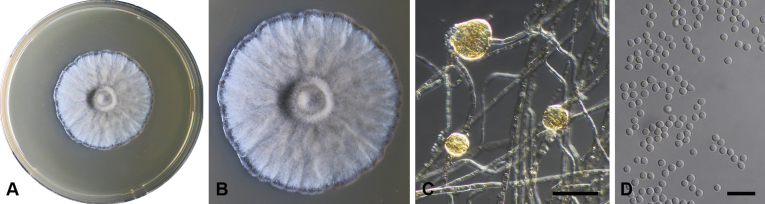
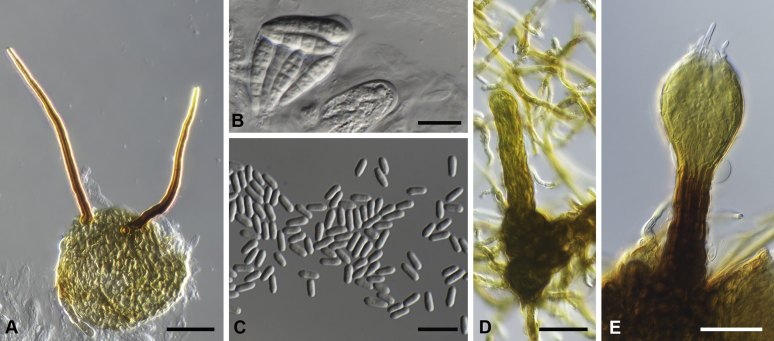
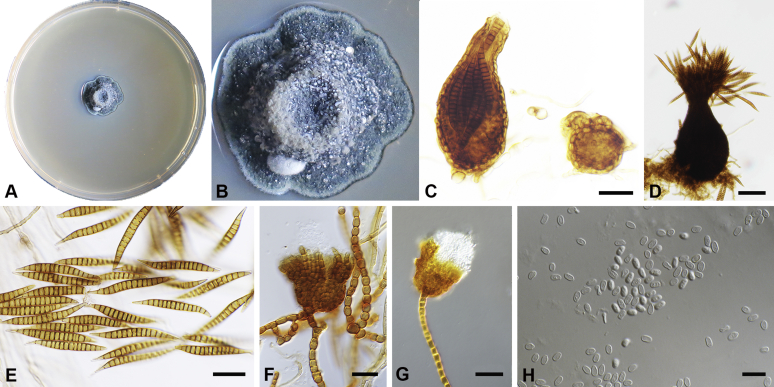
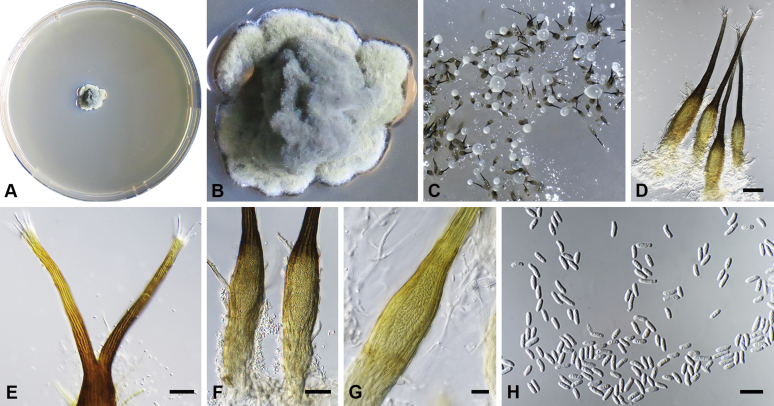
Capnodium alfenasii Abdollahz. & Crous, sp. nov. MycoBank MB833150. Fig. 4.
Fig. 4.
Capnodium alfenasii. A, B. Colony (2-wk-old) on MEA. C. Conidiomata arising from mycelia or immature conidiomata on SNA. D. Conidioma on SNA. E. Ostiole surround by hyaline hyphae. F. Conidia produced in ellipsoidal central part of conidioma. G. Conidia. Scale bars: C = 25 μm; D, E = 20 μm; F, G = 10 μm.
Etymology: Named after Prof. Acelino Couto Alfenas, in recognition to his contributions to the study of Brazilian fungal biodiversity.
Mycelium superficial or immersed, hyaline to brown, branched, hyphae smooth, thin-walled, septate, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial or immersed, flask-shaped, mostly simple and rarely branched, occur singly or in groups, medium to dark brown, synnematous, 113–243 μm long (av. = 187 μm, n = 20), mostly sessile or with short stalk (25–46 × 18–35 μm, av. = 40 × 24 μm), oval or ellipsoidal central part (60–124 × 31–46 μm, av. = 93 × 34 μm), neck (38–118 × 8.8–13.5 μm, av. = 70 × 11 μm), wall comprising mostly cylindrical cells. Ostiole at apex of pycnidia, surrounded by hyaline hyphae. Conidia small, hyaline, aseptate, oblong to ellipsoid, continuous, (3.7–)3.9–4.8(–5.1) × (1.4–)1.7–2(–2.2) μm (av. = 4.4 × 1.8 μm, n = 50).
Culture characteristics: Colonies leathery, appressed, with fluffy aerial mycelium, with creamy exudates of pycnidia containing conidia, folded, edge sinuate, glaucous grey to olivaceous grey after 2 wk in the dark at 25 °C. Colonies reaching 19 mm diam on MEA after 2 wk in the dark at 25 °C.
Typus: Brazil, Minas Gerais, Viçosa, on leaves of Tabebuia sp., 1993, A.C. Alfenas (holotype CBS H-24256, culture ex-type CBS 146151 = CPC 22666.
Additional material examined: Brazil, Minas Gerais, Viçosa, on leaves of Tabebuia sp., 1993, A.C. Alfenas, culture CBS 146152 = CPC 22667, CBS H-24262).
Notes: Phylogenetically Ca. alfenasii forms a distinct clade (Figs S1, 2), but morphologically it is difficult to distinguish from Ca. gamsii, despite having smaller conidia and a shorter central pycnidial body. Ca. alfenasii differs from Ca. blackwelliae in having longer pycnidia, from Ca. neocoffeicola in having smaller pycnidia, and from Ca. paracoffeicola in having smaller pycnidia and conidia.
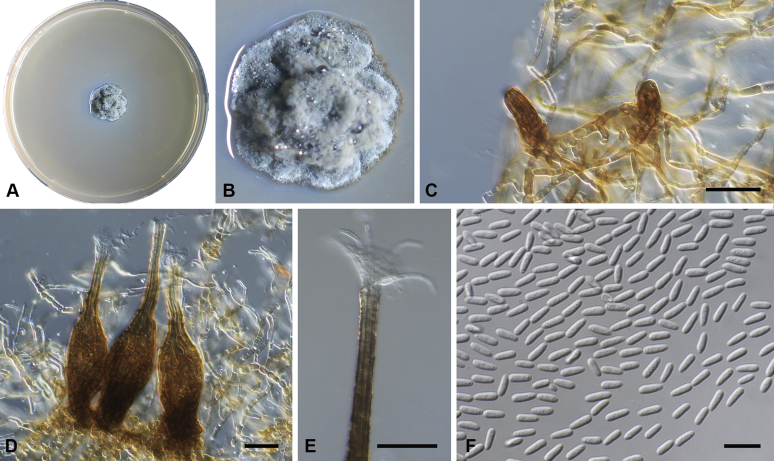
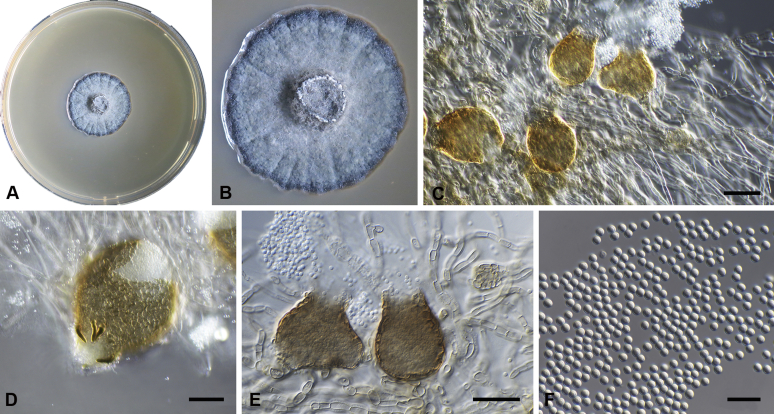
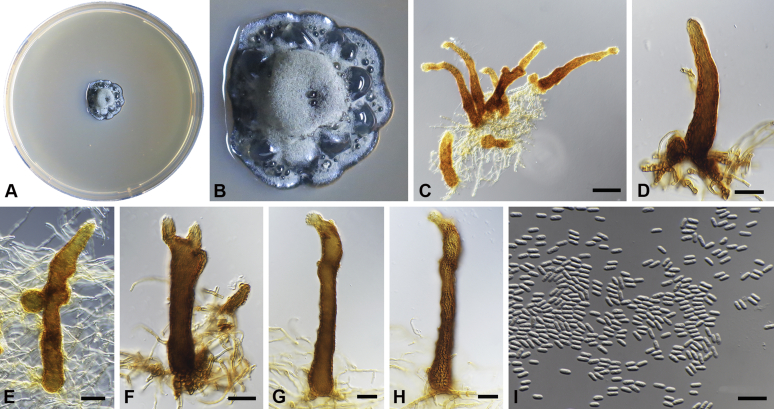
Capnodium blackwelliae Abdollahz. & Crous, sp. nov. MycoBank MB833151. Fig. 5.
Fig. 5.
Capnodium blackwelliae. A, B. Colony (2-wk-old) on MEA. C–G. Conidiomata on SNA. H. Conidia. Scale bars: C–G = 25 μm; H = 10 μm.
Etymology: Named after Prof. Meredith Blackwell, who organised the annual meeting of the Mycological Society of America at Baton Rouge, Louisiana in 2007, during which time this fungus was collected.
Mycelium superficial or immersed, hyaline to brown, branched, hyphae smooth, thin-walled, septate, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial or immersed, slender or flask-shaped, simple or branched, occur singly or in groups, medium to dark brown, synnematous, 42–116 μm long (av. = 95 μm, n = 20), mostly sessile or with short stalk (25–31 × 17–22 μm, av. = 27 × 19 μm), with or without conspicuous oval or ellipsoidal central region (24–143 × 19–55 μm, av. = 70 × 38 μm), with or without neck (22–102 × 10–37 μm, av. = 38 × 12 μm); wall comprising mostly cylindrical cells. Ostiole at pycnidial apex, surrounded by hyaline hyphae. Conidia small, hyaline, aseptate, oblong to ellipsoid, continuous, (3.6–)3.9–4.3(–4.8) × (1.4–)1.6–1.9(–2) μm (av. = 4.2 × 1.7 μm, n = 50).
Culture characteristics: Colonies, leathery, appressed, with fluffy aerial mycelium, with creamy conidial exudates from pycnidia; surface folded, edge metallic, sinuate, greenish glaucous to olivaceous black after 2 wk in the dark at 25 °C. Colonies reaching 22 mm diam on MEA after 2 wk in the dark at 25 °C.
Typus: USA, Louisiana, Baton Rouge, on living leaves of Myrtus communis, 3 Aug. 2007, P.W. Crous (holotype CBS H-24266, culture ex-type CBS 133588).
Notes: Phylogenetically Ca. blackwelliae is closely related to Ca. gamsii (Figs S1, 2), but morphologically differs from all other species by having the smallest pycnidial lengths (av. = 95 μm long). Pycnidia in Ca. blackwelliae are variable in shape, and range from long and flask-shaped to short and cylindrical.
Capnodium coartatum Chomnunti & K.D. Hyde, Fungal Diversity 51: 117. 2011.
Material examined: Thailand, Chiang Rai, on living leaves of Alstonia scholaris, 13 Sep. 2009, K.D. Hyde, culture CPC 17779.
Notes: We examined isolate CPC 17779 and generated sequences of four loci, namely LSU, ITS, TEF-1α and RPB2. This isolate grouped with two isolates of Ca. coartatum, namely MFLUCC10-0069 (ex-type) and MFLUCC10-0070 (Figs S1, 2). There are only LSU sequence data available for both isolates, and they differ at two nucleotide positions. Isolate CPC 17779 is 100 % identical with isolate MFLUCC10-0070 based on LSU sequence data. Morphologically, pycnidia (115–203 μm high, n = 20) and conidia (3.6–4.6 × 1.7–2.5 μm; av. = 3.9 × 2.25 μm, n = 50) of CPC 17779 are both smaller than in Ca. coartatum as described by Chomnunti et al. (2011), although measurements in the latter were made from fungal structures in vivo.
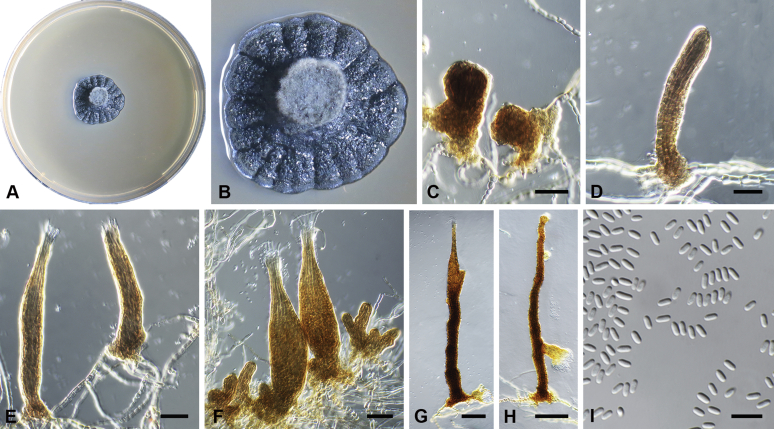
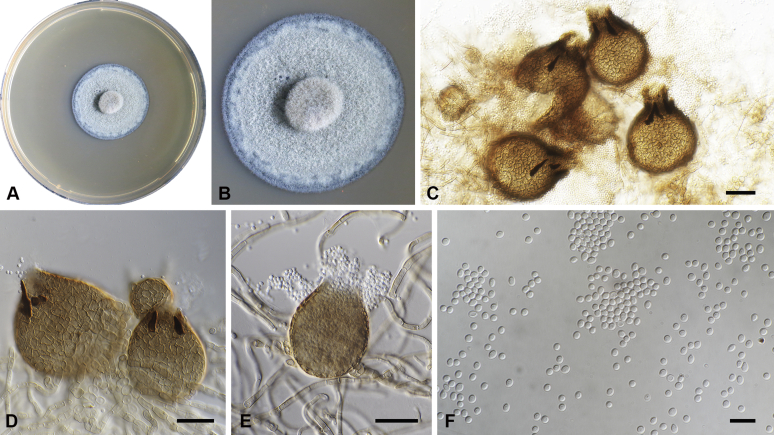
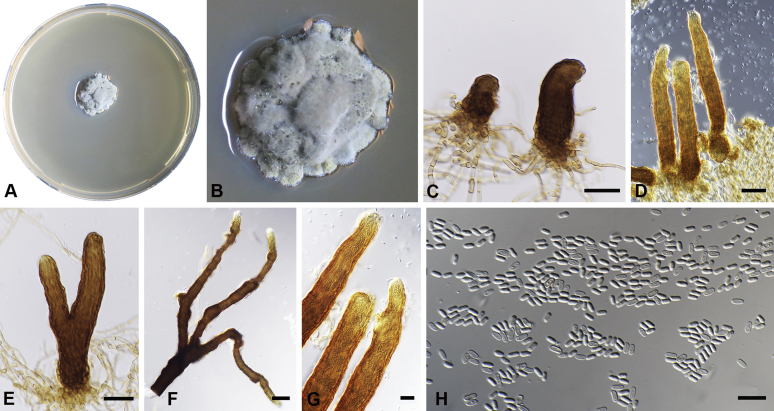
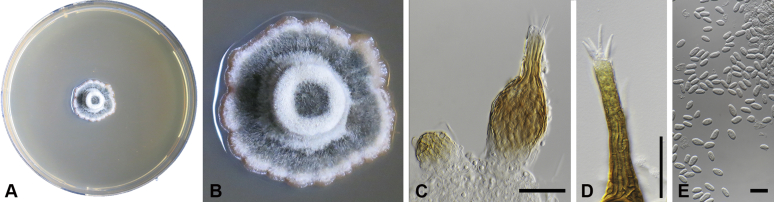
Capnodium gamsii Abdollahz. & Crous, sp. nov. MycoBank MB833152. Fig. 6.
Fig. 6.
Capnodium gamsii. A, B. Colony (2-wk-old) on MEA. C, D. Conidiomata arising from mycelia or immature conidiomata on SNA. E–H. Conidiomata on SNA. I. Conidia. Scale bars: C–F = 25 μm; G, H = 50 μm; I = 10 μm.
Etymology: Named in honour of Prof. K. Walter Gams, who was an avid collector of microfungi, and collected this species in Sri Lanka.
Mycelium superficial or immersed, hyaline to brown, branched, hyphae smooth, thin-walled, septate, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial or immersed, mostly slender or flask-shaped, simple or branched, occur singly or in groups, medium to dark brown, synnematous, 97–350 μm long (av. = 185 μm, n = 20), mostly sessile or with short stalk (23–44 × 16–29 μm, av. = 32 × 21 μm), with or without conspicuous oval or ellipsoidal central part, 62–206 × 19–46 μm, av. = 165 × 40 μm, neck present or absent, 15–87 × 9–25 μm, av. = 40 × 16 μm; wall comprising mostly cylindrical cells. Ostiole at pycnidial apex, surrounded by hyaline hyphae. Conidia small, hyaline, aseptate, oblong to ellipsoid, continuous, (3.6–)4–5.5(–8.1) × (1.6–)1.9–2.4(–2.9) μm (av. = 4.9 × 2.2 μm, n = 50).
Culture characteristics: Colonies, leathery, metallic, appressed, with fluffy aerial mycelium at the centre, with creamy exudates of pycnidia containing conidia, folded, edge sinuate, greenish grey to greenish black after 2 wk in the dark at 25 °C. Colonies reaching 20–22 mm diam on MEA after 2 wk in the dark at 25 °C.
Typus: Sri Lanka, Hakgala Botanic Gardens, on leaves of unknown plant, Jan. 1973, W. Gams (holotype CBS H-24296, culture ex-type CBS 892.73).
Additional materials examined: Thailand, Chiang Rai, on Lagerstroemia speciosa, 1 Jan. 2009, P.W. Crous, culture CPC 17765 = CBS 146153; Chiang Rai, on Lagerstroemia floribunda, 2009, P.W. Crous, cultures CPC 20466 = CBS 146154 and CPC 20467 = CBS 146155); unknown substrate, 2009, P.W. Crous, culture CPC 20471 = CBS 146156, CBS H-24263).
Notes: Capnodium gamsii forms a well-supported phylogenetic clade (Figs S1, 2). Morphologically it is distinguishable from other species in having more cylindrical pycnidia with a much longer (av. = 165 × 40 μm, l/w ratio > 4) central region. In other species the average length of the central region is less than 100 μm (l/w ratio < 3).
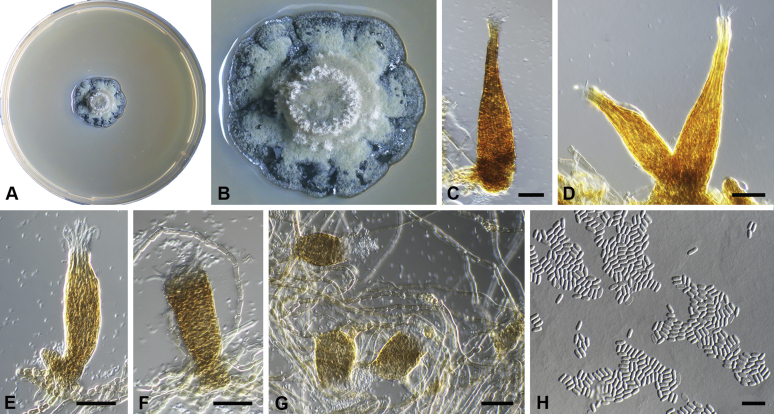
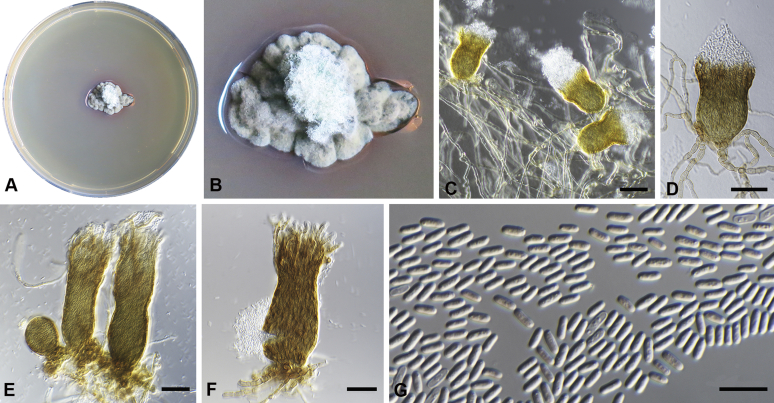
Capnodium neocoffeicola Abdollahz. & Crous, sp. nov. MycoBank MB833153. Fig. 7.
Fig. 7.
Capnodium neocoffeicola. A, B. Colony (2-wk-old) on MEA. C. Conidiomata arising from mycelia or immature conidiomata on SNA. D. Conidiomata on SNA. E. Conidia produced in ellipsoidal central part of conidioma. F. Conidia. Scale bars: C, E = 20 μm; D = 25 μm; F = 10 μm.
Etymology: Name refers to the fact that it is related to Ca. coffeicola.
Mycelium superficial or immersed, hyaline to brown, branched; hyphae smooth, thin-walled, septate, constricted at septa, with a mucilaginous outer wall layer. Conidiomata pycnidial, superficial or immersed, flask-shaped, simple and erect, occur singly or in groups, medium to dark brown, synnematous, 134–268 μm long (av. = 230 μm, n = 20), sessile or with short stalk (27–46 × 28–40 μm, av. = 38 × 30 μm), oval or ellipsoidal central part (74–115 × 38–52 μm, av. = 90 × 46 μm), neck (44–136 × 9–12 μm, av. = 110 × 11 μm), wall comprising mostly cylindrical cells. Ostiole at apex of pycnidial neck, surrounded by hyaline hyphae. Conidia small, hyaline, aseptate, oblong to ellipsoid, continuous, (3.7–)4–4.7(–5.2) × (1.6–)1.8–2(–2.3) μm (av. = 4.4 × 1.9 μm, n = 50).
Culture characteristics: Colonies leathery, appressed, with fluffy aerial mycelium, with abundant creamy exudates of pycnidia containing conidia, folded, edge sinuate, glaucous grey to pale greenish grey after 2 wk in the dark at 25 °C. Colonies reaching 19–22 mm diam on MEA after 2 wk in the dark at 25 °C.
Typus: Thailand, Chiang Rai, on living leaves of Coffea arabica, 20 Aug. 2014, S. Hongsanan (holotype CBS H-24267, culture ex-type CBS 139614 = MFLUCC 14-0570).
Additional material examined: Thailand, Chiang Rai, on living leaves of Coffea arabica, 20 Aug. 2014, S. Hongsanan, culture CBS 139613 = MFLUCC 14-0569.
Notes: In the phylogenetic tree, Ca. neocoffeicola is clearly a distinct species (Figs S1, 2). In terms of morphology, the smaller conidia can differentiate Ca. neocoffeicola from Ca. paracoffeicola, and the longer pycnidia (av. = 230 μm) distinguishes it from other species examined in this study. In Ca. paracoffeicola, Ca. neocoffeicola and Ca. coffeae (CBS 147.52) the average pycnidial length is greater than 200 μm, while in the other species (incl. Ca. coffeicola, 165–178 μm) pycnidia are less than 200 μm long.
Capnodium paracoffeicola Abdollahz. & Crous, sp. nov. MycoBank MB833154. Fig. 8.
Fig. 8.
Capnodium paracoffeicola. A, B. Colony (2-wk-old) on MEA. C. Conidiomata arising from mycelia or immature conidiomata on SNA. D. Conidiomata on SNA. E. Ostiole surround by hyaline hyphae. F. Conidia. Scale bars: C–E = 25 μm; F = 10 μm.
Etymology: Name refers to the fact that it is related to Ca. coffeicola.
Mycelium superficial or immersed, hyaline to brown, branched; hyphae smooth, thin-walled, septate, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial or immersed, flask-shaped, simple or branched, occurring singly or in groups, medium to dark brown, synnematous, 223–337 μm long (av. = 266 μm, n = 20), sessile or with short stalk (32–87 × 17–28 μm, av. = 70 × 23 μm), oval or ellipsoidal central part (63–160 × 25–40 μm, av. = 90 × 35 μm), neck (82–173 × 8.8–13.9 μm, av. = 120 × 12 μm), wall comprising mostly cylindrical cells. Ostiole at apex of pycnidial neck, surrounded by hyaline hyphae. Conidia small, hyaline, aseptate, oblong to ellipsoid, continuous, (4.9–)5–6.5(–7.7) × (1.8–)1.9–2.3(–2.6) μm (av. = 6.4 × 2.15 μm, n = 50).
Culture characteristics: Colonies leathery, appressed, with fluffy aerial mycelium, folded, edge sinuate, glaucous grey to pale greenish grey after 2 wk in the dark at 25 °C. Colonies reaching 17 mm diam on MEA after 2 wk in the dark at 25 °C.
Typus: Thailand, Chiang Rai, on living leaves of Coffea arabica, 20 Aug. 2014, S. Hongsanan (holotype CBS H-24268, culture ex-type CBS 139616 = MFLUCC 14-0572).
Additional material examined: Thailand, Chiang Rai, on living leaves of Coffea arabica, 20 Aug. 2014, S. Hongsanan, culture CBS 139615 = MFLUCC 14-0571.
Notes: Phylogenetically this species constitutes a distinct clade (Figs S1, 2) that is characterised morphologically by larger conidia (av. = 6.4 × 2.15 μm). Average conidial lengths of the other species studied here are shorter than 5 μm. Capnodium coffeicola was recently described from leaves of Coffea sp. collected in Chiang Rai, Thailand (Hongsanan et al. 2015b). Morphologically it differs from Ca. paracoffeicola in its shorter conidiomata (165–178 μm long), and shorter stalks (19–24 long × 18–23 μm diam; Hongsanan et al. 2015b).
Chaetocapnodium Hongsanan & K.D. Hyde, Fungal Diversity 72: 68. 2015.
Type species: Chaetocapnodium siamensis Hongsanan & K.D. Hyde
Notes: Chaetocapnodium is a hitherto monotypic genus introduced based on the morphology of its sexual morph and supported by LSU sequence data (Liu et al. 2015). The phylogenies generated in the present study (Figs S1, 2), however, revealed that Antennariella placitae and Phragmocapnias philippinensis are congeneric with Chaetocapnodium. Nine other isolates clustered in six distinct clades representing six species, five of which are recognised as taxonomic novelties. Two new combinations are proposed and five new species described in Chaetocapnodium.
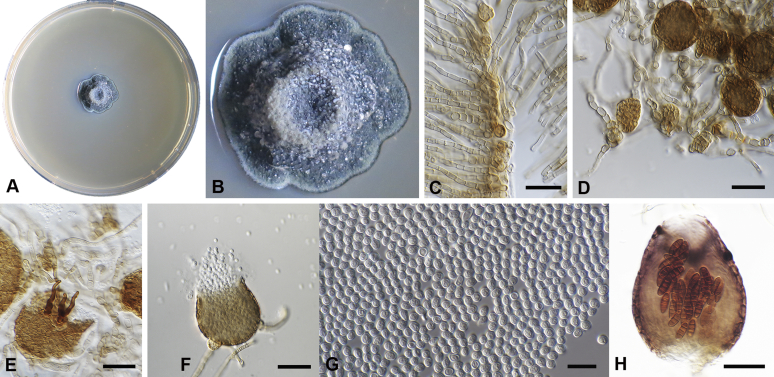
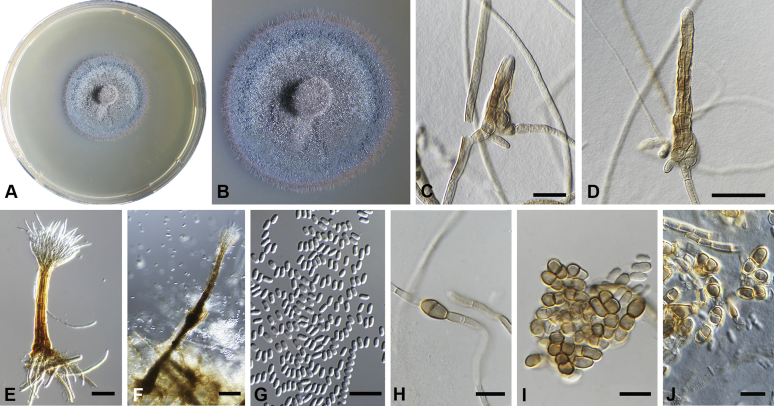
Chaetocapnodium indonesiacum Abdollahz. & Crous, sp. nov. MycoBank MB833156. Fig. 9.
Fig. 9.
Chaetocapnodium indonesiacum. A, B. Colony (2-wk-old) on MEA. C. Conidiomata on SNA. D. Conidia. Scale bars: C = 25 μm; D = 10 μm.
Etymology: Name refers to Indonesia where this fungus was collected.
Mycelium superficial or immersed, hyaline to medium brown, branched, hyphae mostly smooth, thin-walled, septate, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial or immersed, globose to pyriform, pale to dark brown, mostly intercalary, lateral or terminal on erect hyphal branches, meristogenous in development, pseudoparenchymatous, thin-walled, 1–2 cell layers of textura angularis, (20–)25–35(–48) × (16–)23–33(–40) μm. Setae not observed. Ostiole absent, or not well-developed, mostly releasing conidia by means of irregular rupture. Conidia hyaline, aseptate, globose to subglobose, with minute guttules, smooth, thin-walled, (2–)2.4–2.8 × (1.8–)2.2–2.4(–2.6) μm, (av. = 2.5 × 2.2 μm, n = 50).
Culture characteristics: Colonies, leathery, appressed, with fluffy aerial mycelium, folded, edge sinuate, smoke grey to pale mouse grey after 2 wk in the dark at 25 °C. Colonies reaching 43 mm diam on MEA after 2 wk in the dark at 25 °C.
Typus: Indonesia, Java Island, Bogor, Buitenzorg, on leaves of Camellia sinensis, 1930?, F.H. van Beyma (holotype CBS H-24269, culture ex-type CBS 202.30).
Notes: Phylogenetically Ch. indonesiacum clusters with Ch. philippinense (Figs S1, 2). Morphologically it is not possible to compare these two species, as the latter is known only from its sexual morph. They are genetically distinct in 1, 1, 9 and 40 bp in LSU, ITS, TEF-1α and RPB2 loci. Chaetocapnodium indonesiacum can be distinguished from all other species by having the smallest pycnidia (25–35 × 23–33 μm). With the excepton of Ch. indonesiacum and Ch. placitae, all Chaetocapnodium species have conidiomatal pycnidia with setae. The radial growth rate of Ch. indonesiacum on MEA at 25 °C was more rapid (43 mm diam/2 wk) than that observed in all other species.
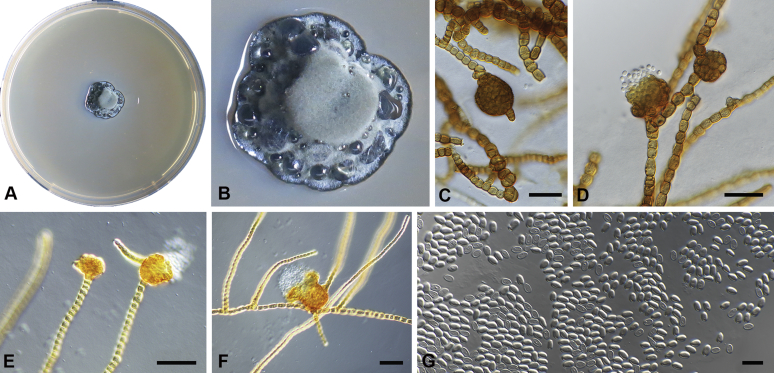
Chaetocapnodium insulare Abdollahz. & Crous, sp. nov. MycoBank MB833157. Fig. 10.
Fig. 10.
Chaetocapnodium insulare. A, B. Colony (2-wk-old) on MEA. C, D. Septate hyphae with mucilaginous outer wall layer and immature conidiomata on SNA. E. Conidiomata with setae. F. Conidia inside conidioma. G. Conidia. H. Ascoma with 3-septate brown ascospores. Scale bars: C–F, H = 20 μm; G = 10 μm.
Etymology: Name reflects the fact that it was collected from an island.
Mycelium superficial or immersed, hyaline to medium brown, branched, hyphae mostly smooth, thin-walled, septate, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial or immersed, globose to pyriform, pale to dark brown, mostly intercalary, lateral or terminal on erect hyphal branches, meristogenous in development, pseudoparenchymatous, thin-walled, 1–2 cell layers of textura angularis, (28–)35–55 × (22–)30–48 μm. Setae present, septate or aseptate, pale to dark brown, mostly around ostiole, (7–)10–13(–19) μm long (av. = 12 μm, n = 30). Ostiole absent, or not well-developed, mostly releasing conidia by means of irregular rupture. Conidia hyaline, aseptate, globose to subglobose, with minute guttules, smooth, thin-walled, (2.8–)3.2–3.6(–4.4) × (2.6–)2.9–3.3(–3.7) μm, (av. = 3.4 × 3 μm, n = 50).
Culture characteristics: Colonies, leathery, appressed, with fluffy aerial mycelium, with creamy conidial exudates, edge sinuate, smoke grey to greenish grey after 2 wk in the dark at 25 °C. Colonies reaching 14 mm diam on MEA after 2 wk in the dark at 25 °C.
Typus: South Africa, Marion Island, Prince Edward Is., on Phylica arborea, 2011, M.J. Wingfield (holotype CBS H-24297, culture ex-type CPC 19221 = CBS 146159).
Additional material examined: South Africa, Marion Island, Prince Edward Is., on P. arborea, 2011, M.J. Wingfield, culture CPC 19223 = CBS 146160; on P. arborea, 2011, M.J. Wingfield, culture CPC 19224 = CBS 146161.
Notes: Based on the phylogenetic analyses Ch. insulare is related to Ch. placitae (Figs S1, 2), but morphologically it is distinct from all Chaetocapnodium species examined in this study by producing the largest conidia with average length and width greater than 3 (av. = 3.4 × 3 μm). Moreover, pycnidia in Ch. insulare are setose with septate or aseptate setae while in Ch. placitae setae are absent. Radial growth rate on MEA at 25 °C is slower (14 mm diam/2 wk) than observed for all other species.
Chaetocapnodium philippinense (Hongsanan & K.D. Hyde) Abdollahz. & Crous, comb. nov. MycoBank MB833158.
Basionym: Phragmocapnias philippinensis Hongsanan & K.D. Hyde, Fungal Diversity 72: 69. 2015.
Description: Liu et al. (2015).
Typus: Philippines, Laguna, Mount Makiling, on leaves of palm (Arecaceae), Feb. 2012, K.D. Hyde HSA14/1 (holotype MFLU 14-0748, ex-type culture MFLUCC 12-0110 = CPC 20474).
Chaetocapnodium placitae (Cheewangkoon & Crous) Abdollahz. & Crous, comb. nov. MycoBank MB833159.
Basionym: Antennariella placitae Cheewangkoon & Crous, Persoonia 23: 57. 2009.
Description: Cheewangkoon et al. (2009).
Typus: Australia, New South Wales, Cessnock S 32°50′45″, E 151°17′07″, on Eucalyptus placita, 14 Oct. 2006, coll. B.A. Summerell, isol. P.W. Crous (holotype CBS H-20277, culture ex-type CPC 13706 = CBS 124785).
Chaetocapnodium siamensis Hongsanan & K.D. Hyde, Fungal Diversity 72: 69. 2015.
Asexual morph. Mycelium superficial or immersed, hyaline to medium brown, branched, hyphae mostly smooth, thin-walled, septate, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial or immersed, globose to pyriform, pale to dark brown, mostly intercalary, lateral or terminal on erect hyphal branches, meristogenous in development, pseudoparenchymatous, thin-walled, 1–2 cell layers of textura angularis, (36–)45–70(–100) × (32–)40–65(–94) μm. Setae present, septate or aseptate, pale to dark brown, mostly around ostiole, (13.9–)20–26(–30) μm long (av. = 22.7 μm, n = 30). Ostiole absent, or not well-developed, mostly releasing conidia by means of irregular rupture. Conidia hyaline, septate or aseptate, globose to subglobose, with minute guttules, smooth, thin-walled, (2.1–)2.4–2.8(–3) × 2–2.4(–2.6) μm (av. = 2.6 × 2.3 μm, n = 50). For description of sexual morph, see Liu et al. (2015).
Material examined: Thailand, Chiang Rai, Bandu, on leaves of unknown plant host, 2013, S.C. Karunarathna, culture CBS 139815 = MFLUCC 13-0096.
Notes: Isolate CBS 139815 clustered (Figs S1, 2) with the ex-type of Ch. siamensis (MFLUCC13-0778, on an unidentified host plant, collected in Chiang Rai). Only LSU sequence data are available for Ch. siamensis, which differs from CBS 139815 at two nucleotide positions. We have characterised the asexual morph of CBS 139815, which was not described in the original description of Ch. siamensis.
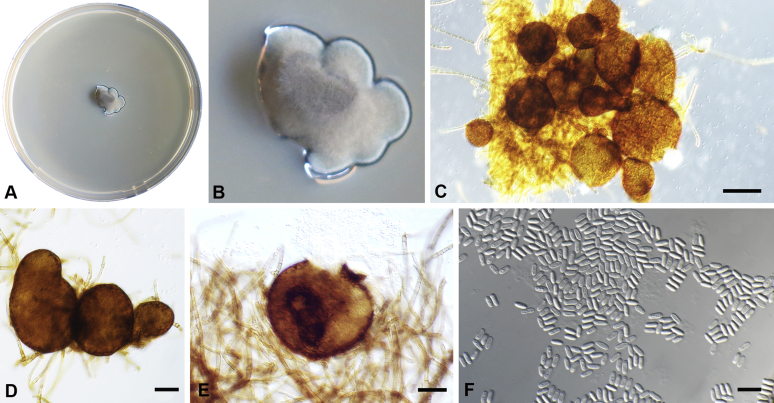
Chaetocapnodium summerellii Abdollahz. & Crous, sp. nov. MycoBank MB833155. Fig. 11.
Fig. 11.
Chaetocapnodium summerellii. A, B. Colony (2-wk-old) on MEA. C. Conidiomata on SNA. D. Conidioma with setae. E. Conidia inside conidiomata. F. Conidia. Scale bars: C, D = 25 μm; E = 20 μm; F = 10 μm.
Etymology: Named in honour of Prof. Brett A. Summerell, Director Research & Chief Botanist at the Royal Botanic Garden Sydney, Australia, who is an active advocate for plant and fungal conservation.
Mycelium superficial or immersed, hyaline to medium brown, branched, hyphae mostly smooth, thin-walled, septate, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial or immersed, globose to pyriform, pale to dark brown, mostly intercalary, lateral or terminal on erect hyphal branches, meristogenous in development, pseudoparenchymatous, thin-walled, 1–2 cell layers of textura angularis, (33–)40–60(–67) × (27–)40–55(–60) μm. Setae present, septate or aseptate, pale to dark brown, mostly around ostiole, (9–)11–15(–19) μm long (av. = 14.3 μm, n = 30). Ostiole absent or not well-developed, mostly releasing conidia by means of irregular rupture. Conidia hyaline, aseptate, globose to subglobose, with minute guttules, smooth, thin-walled, (2–)2.2–2.5(–2.6) × 1.9–2.4 μm, (av. = 2.3 × 2.1 μm, n = 50).
Culture characteristics: Colonies, leathery, appressed, with fluffy aerial mycelium, with creamy conidial exudates in centre of colony; surface folded, edge sinuate, pale mouse grey to olivaceous black after 2 wk in the dark at 25 °C. Colonies reaching 29 mm diam on MEA after 2 wk in the dark at 25 °C.
Typus: Australia, New South Wales, on leaves of Eucalyptus placita (Myrtaceae), Oct. 2006, B.A. Summerell (holotype CBS H-24257, culture ex-type CPC 13654 = CBS 146157).
Additional material examined: Laos, host unknown, 1 Jan. 2009, P. Pheng, culture CPC 17368 = CBS 146158, CBS H-24264.
Notes: Chaetocapnodium summerellii resembles Ch. thailandense and Ch. tanzanicum in morphology, but is phylogenetically distinct, forming a separate clade (Figs S1, 2). Furthermore, its radial growth rate on MEA at 25 °C (29 mm diam/2 wk) is slower than that of the latter two species (38 mm diam/2 wk).
Chaetocapnodium tanzanicum Abdollahz. & Crous, sp. nov. MycoBank MB833160. Fig. 12.
Fig. 12.
Chaetocapnodium tanzanicum. A, B. Colony (2-wk-old) on MEA. C, D. Conidia inside conidiomata. E, F. Conidiomata with setae on SNA. G. Conidia. Scale bars: C, D, F = 25 μm; E = 50 μm; G = 10 μm.
Etymology: Name refers to Tanzania where this fungus was collected.
Mycelium superficial or immersed, hyaline to medium brown, branched, hyphae mostly smooth, thin-walled, septate, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial or immersed, globose to pyriform, pale to dark brown, mostly intercalary, lateral or terminal on erect hyphal branches, meristogenous in development, pseudoparenchymatous, thin-walled, 1–2 cell layers of textura angularis, (38–)55–70(–138) × (30–)45–65(–112) μm. Setae present, septate or aseptate, pale to dark brown, mostly around ostiole, (10.5–)12–17(–23) μm long (av. = 15.6 μm, n = 30). Ostiole absent or not well-developed, mostly releasing conidia by means of irregular rupture. Conidia hyaline, aseptate, globose to subglobose, with minute guttules, smooth, thin-walled, (2.5–)2.7–2.9(–3.2) × (2.3–)2.5–2.8 μm, (av. = 2.8 × 2.6 μm, n = 50).
Culture characteristics: Colonies, leathery, metallic, appressed, with fluffy aerial mycelium at the centre, with creamy exudates of pycnidia containing conidia, folded, edge sinuate, smoke grey to greenish black, edge sienna to cinnamon after 2 wk in the dark at 25 °C. Colonies reaching 38 mm diam on MEA after 2 wk in the dark at 25 °C.
Typus: Tanzania, on lichen, 1974, M. Dreyfuss (holotype CBS H-24270, culture ex-type CBS 145.79).
Notes: Chaetocapnodium tanzanicum is phylogenetically clearly distinct from other Chaetocapnodium spp. (Figs S1, 2). Morphologically it resembles Ch. thailandense and Ch. summerellii. It is distinguishable from Ch. summerellii by having a faster radial growth rate on MEA at 25 °C, and from Ch. thailandense by producing larger conidia.
Chaetocapnodium thailandense Abdollahz. & Crous, sp. nov. MycoBank MB833161. Fig. 13.
Fig. 13.
Chaetocapnodium thailandense. A, B. Colony (2-wk-old) on MEA. C, D. Conidiomata with setae on SNA. E. Conidia inside conidioma. F. Conidia. Scale bars: C = 25 μm; D, E = 20 μm; F = 10 μm.
Etymology: Name refers to Thailand where this fungus was collected.
Mycelium superficial or immersed, hyaline to medium brown, branched, hyphae mostly smooth, thin-walled, septate, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial or immersed, globose to pyriform, pale to dark brown, mostly intercalary, lateral or terminal on erect hyphal branches, meristogenous in development, pseudoparenchymatous, thin-walled, 1–2 cell layers of textura angularis, (32–)40–70(–90) × (32–)40–60(–80) μm. Setae present, aseptate, pale to dark brown, mostly around ostiole, (8.7–)13–19(–26.6) μm long (av. = 16.1 μm, n = 30). Ostiole absent, or not well-developed, mostly releasing conidia by means of irregular rupture. Conidia hyaline, 0(–1)-septate, globose to subglobose, with minute guttules, smooth, thin-walled, (2–)2.2–2.6(–2.8) × (1.9–)2–2.7 μm (av. = 2.3 × 2.1 μm, n = 50).
Culture characteristics: Colonies, leathery, appressed, with fluffy aerial mycelium, glaucous grey to pale olivaceous grey after 2 wk in the dark at 25 °C. Colonies reaching 38 mm diam on MEA after 2 wk in the dark at 25 °C.
Typus: Thailand, Chiang Rai, host plant unknown, 2013, S.C. Karunarathna (holotype CBS H-24271, culture ex-type CBS 139619 = MFLUCC 13-0787).
Notes: Phylogenetically, Ch. thailandense constitutes a distinct lineage (Figs S1, 2). Morphologically Ch. thailandense resembles Ch. tanzanicum and Ch. summerellii. It is distinguished from Ch. tanzanicum by having smaller conidia, and from Ch. summerellii by its faster radial growth rate on MEA at 25 °C.
Conidiocarpus Woron., Ann. Mycol. 24 (3/4): 250. 1926.
Saprobic on sugary exudates from insects, with dark mycelium forming a soot-like coating on the upper surface of leaves. Thallus composed of black, pelliculose, reticulately branched, dense, cylindrical, radiating, septate hyphae. Ascomata not observed. Conidiomata pycnidial, supported on black, long, narrow, cylindrical stalks composed of tightly compacted, anastomosed, synnematous cylindrical hyphae, lageniform with a brown oval or ellipsoid part, which produces a long neck and conidia. The pycnidium wall is composed of two or more layers, the outer one being more or less pseudoparenchymatous although the short cells tend to be arranged linearly, indicating their origin from longitudinally fused hypha. Ostiole surrounded by hyaline, subulate, hyphal extensions. Conidia small, ellipsoid, continuous, aseptate, hyaline, smooth-walled, arranged in a droplet at the apex of pycnidial neck (adapted from Hughes, 1976, Chomnunti et al., 2011).
Type species: Conidiocarpus caucasicus Woron.
Notes: Conidiocarpus with the type species Co. caucasicus was introduced by Woronichin in Jaczewski (1917). However, Hughes (1976) stated that Batista & Ciferri (1963a) considered Co. penzigii, the second oldest species introduced in 1926, as the type species. Conidiocarpus has been reported as the asexual morph of Phragmocapnias (Hughes 1976). Phragmocapnias betle is the type species of Phragmocapnias. Following the ICN code based on the priority rule and one fungus = one name principles, Bose et al. (2014) chose Conidiocarpus and transferred species of Phragmocapnias to Conidiocarpus.
In this study based on phylogenetic analyses, the type species of Conidiocarpus and Phragmocapnias clustered in two distinct clades representing two different genera (Figs S1, 2). As discussed by Hughes (1976), pycnidia of Co. caucasicus are elongated, 540–650 μm long including a stalk, swollen part and a neck. In morphological studies, we found that all of the species that grouped with C. caucasicus produced a Conidiocarpus pycnidial type, typified by having a long neck (Fig. 14).
Fig. 14.
Conidiocarpus, Phragmocapnias and Polychaeton conidiomata. A, B.Conidiocarpus conidiomata on SNA. C–E.Conidiocarpus conidiomata on OA. F.Polychaeton conidioma on SNA. G, H.Polychaeton conidiomata on OA. I, J.Phragmocapnias conidiomata on SNA. Scale bars: A, C–E, G, H = 50 μm; F, J = 25 μm; I = 20 μm.
Hughes (1976) mentioned that in cases where the sexual-asexual connections have been confirmed, the pycnidial morphs of Phragmocapnias were the tall Conidiocarpus conidiomata that lacked necks (Fig. 14, Fig. 16). Chomnunti et al. (2011) designated an epitype (MFLU09-0650, living culture MFLUCC10-0053) for the type species Phragmocapnias betle, and re-described this species based on the sexual morph. They did not observe the asexual morph. Based on Hughes (1976), asexual conidiomata are Conidiocarpus pycnidia (150–700 μm long) that lack a neck. To observe the pycnidial morph of Phragmocapnias betle, we used different culture media and were able to introduce the asexual morph on PDA, PCA and CMA. Conidiomata were pycnidia with short stalks, ellipsoidal swellings, and lacking necks. Therefore, following the views of Hughes (1976), we chose to resurrect Phragmocapnias for species with conidiocarpus-like pycnidia lacking necks.
Fig. 16.
Phragmocapnias betle. A. Ascoma with setae on SNA. B. Asci and ascospores. C. Conidia. D. Conidioma arising from mycelium on SNA. E. Conidioma on SNA. Scale bars: A, D = 25 μm; E = 20 μm; B, C = 10 μm.
A search of Index Fungorum and MycoBank (March 2019) revealed 12 names in Conidiocarpus, of which six species, Co. asiaticus, Co. betle, Co. caucasicus, Co. philippinensis, Co. plumeriae and Co. siamensis have DNA sequence data.
In our phylogenetic analyses based on four loci (LSU, ITS, TEF-1α and RPB2), Conidiocarpus isolates clustered in two subclades (Figs S1, 2). The ex-type isolates of Co. asiaticus and Co. siamensis together with Co. caucasicus and the nine isolates sequenced in this study clustered in the first subclade strongly supported in the RAxML analysis, but with no support from the Bayesian analyses. These species are morphologically different but phylogenetically unresolved, which may be due to missing data. Only LSU sequence data are available for the ex-type strains of Co. caucasicus (GUMH937) and Co. siamensis (MFLUCC10-0064), and LSU/ITS sequences for Co. asiaticus (MFLUCC10-0062). Two isolates CPC 20464 and CPC 20468 for which four genes sequenced in this study and an isolate belonging to Co. siamensis (MFLUCC10-0061) with LSU and ITS sequences clustered in the second sub-clade, representing a putatively new Conidiocarpus species supported by both RAxML and Bayesian analyses (ML-BS = 100 %, PP = 0.82). However, the identity of the other Conidiocarpus isolates included in this study can only be resolved once additional gene regions have been sequenced.
Heteroconium Petr., Sydowia 3: 264. 1949.
Type species: Heteroconium citharexyli Petr.
Descriptions: Hughes, 2007, Cheewangkoon et al., 2012.
Notes: A search of Index Fungorum and MycoBank (March 2019) lists 25 and 28 names in Heteroconium, respectively. However, only LSU sequence data are available for the type species and no sequence data are available for other species in the genus. Heterconium kleinzeense was recently transferred to Blastacervulus (Crous et al., 2019a, Crous et al., 2019b).
Leptoxyphium Speg., Physis, Rev. Soc. Arg. Cienc. Nat. 4 (17): 294. 1918.
Type species: Leptoxyphium graminum (Pat.) Speg.
Notes: A search in Index Fungorum and MycoBank (March 2019) listed 18 names in Leptoxyphium. However, sequence data are available only for the types of four species; L. cacuminum MFLUCC10-0059 (LSU), L. glochidion IFRDCC 2651 (LSU/ITS), L. kurandae CBS 129530 (LSU, ITS/TEF-1α/RPB2) and L. madagascariense CBS 124766 (LSU, ITS/TEF-1α/RPB2). Leptoxyphium cacuminum and L. glochidion are identical based on LSU sequences, but they are morphologically different. These species clustered in the same clade together with 14 isolates considered in this study (Figs S1, 2). Some variation in nucleotide sequences (especially RPB2) was observed within this clade, which may indicate intra- or interspecific variation. Isolates CBS 451.66 and CPC 26196 clustered in a distinct clade (Figs S1, 2) representing a new species described below.
Leptoxyphium citri Abdollahz. & Crous, sp. nov. MycoBank MB833163. Fig. 15.
Fig. 15.
Leptoxyphium citri. A, B. Colony (2-wk-old) on MEA. C, D. Conidiomata arising from mycelia on SNA. E. Mature funnel-shaped conidioma at apex with hyaline hyphae surrounding the ostiole. F. Proliferation through the fertile head of conidioma. G. Conidia. H–J. Synasexual morph 2-celled conidia. Scale bars: C = 40 μm; D = 20 μm; E = 25 μm; F = 50 μm; G–J = 10 μm.
Etymology: Name refers to Citrus, the host genus from which it was collected.
Mycelium superficial or immersed, grey to pale brown, branched, smooth to finely verruculose, thick-walled, septate, constricted at septa, with a mucilaginous outer wall layer, forming hyphal ropes. Conidiomata synnematous, simple or successively proliferating through the fertile head to produce another conidiogenous apex at a higher level, single or in groups, erect, straight to slightly flexuous; bulbous base medium to dark brown, cylindrical part dark olivaceous brown, 53–153 × 6–12 μm, expanding to a funnel-shaped hyphal apex, 20–40 μm high, 15–46 μm wide. Conidiophores subcylindrical to subulate, septate, tightly aggregated in apical part of synnema, among synnematous hyphae that diverge close to apex. Conidiogenous cells integrated, terminal, phialidic, tapering to a truncate apex. Conidia broadly ellipsoid with rounded ends, aseptate, eguttulate, hyaline, smooth, (3.9–)4.3–4.9(–5.3) × (1.9–)2.1–2.4(–2.6) μm (av. = 4.7 × 2.2 μm, n = 50), aggregating in hyaline, slimy masses at apex of synnemata. Synasexual morph conidia arthric, single or in chains, frequently around the bulbous base of the synnemata, cylindrical to ellipsoid or ovoid, 1-septate, constricted at septum, smooth, pale to medium brown, 6–12 × 2–5 μm.
Culture characteristics: Colonies, appressed, with fluffy aerial mycelium, with creamy conidial exudates, smoke grey to pale olivaceous grey after 2 wk in the dark at 25 °C. Colonies reaching 39 mm diam on MEA after 2 wk in the dark at 25 °C.
Typus: Spain, on fruit of Citrus sinensis, Jan. 1966, H.A. van der Aa (holotype CBS H-14520, culture ex-type CBS 451.66).
Additional material examined: Italy, on Citrus sp., 2015, V. Guarnaccia, culture CPC 26196 = CBS 146162, CBS H-24265.
Notes: Phylogenetically, L. citri is closely related to L. kurandae (Figs S1, 2). These two species differ in 1, 4 and 10 bp in LSU, TEF-1α and RPB2 loci, respectively. Conidia of L. citri are smaller (4.3–4.9 × 2.1–2.4 μm) than those of L. kurandae (6–7 × 2–3 μm). It is interesting that both isolates of L. citri are known from Citrus collected in Europe.
Phragmocapnias Theiss. & Syd., Ann. Mycol. 15: 480. 1918.
Saprobic on sugary exudates from insects, dark mycelium forming a soot-like coating on the upper surface of leaves. Thallus composed of black, pelliculose, reticulately branched, dense, cylindrical, radiating, septate hyphae. Ascomata scattered, subglobose to broadly ellipsoidal, barely stalked, firmly attached to the basal hyphae, dark brown, thick-walled, ostiolate, with setae. Peridium consisting of pale to dark brown cells forming a textura angularis. Asci bitunicate, 8-spored, broadly clavate, with short pedicle. Ascospores cylindrical-clavate, hyaline, 4-septate and constricted at the septum (Chomnunti et al. 2011). Conidiomata pycnidial, similar to Conidiocarpus, but with a short stalk and oval or ellipsoid part and ostiole, lacking a neck. Ostiole surrounded by hyaline, subulate hyphal extensions. Conidia small, ellipsoid, continuous, aseptate, hyaline, smooth-walled, arranged in a droplet at the apex of pycnidium (Fig. 14, Fig. 16).
Type species: Phragmocapnias betle (Syd. et al.) Theiss. & Syd.
Notes: Of the six Conidiocarpus species for which DNA sequence data are available, two species, Co. betle and Co. plumeriae, clustered in Phragmocapnias (Figs S1, 2). Phragmocapnias betle is consequently resurrected, and a new combination is introduced for Conidiocarpus plumeriae.
Phragmocapnias betle (Syd. et al.) Theiss. & Syd., Ann. Mycol. 15: 480. 1918. Fig. 16.
Descriptions: Hughes, 1976, Chomnunti et al., 2011.
Notes: Chomnunti et al. (2011) provided a detailed description for P. betle based on the sexual morph, and designated an epitype for the species. The following description is provided for the asexual morph:
Mycelium superficial or immersed, hyaline to brown, branched, hyphae smooth, thin-walled, septate, constricted at septa, with a mucilaginous outer wall layer. Conidiomata pycnidial, brown, comprised of cylindrical septate cells, (67–)90–120(–135) μm high (av. = 105 μm, n = 20), stalk brown to black, (27–)50–70(–97) μm high (av. = 50 μm, n = 20), 12–31 diam (av. = 24 μm, n = 20), the oval, swollen part which produces conidia is brown, comprised of cylindrical, septate cells, (37–)50–60(–69) μm high (av. = 48 μm, n = 20), (30–)40–55(–75) μm diam (av. = 53 μm, n = 20). Ostiole surrounded by hyaline hyphae. Conidiogenous cells formed on the inner layer of the oval part. Conidia oblong to ellipsoid, aseptate, hyaline, continuous, (4–)5–6(–7.9) × (1.4–)1.8–2.2(–2.8) μm (av. = 5.5 × 2 μm, n = 50).
Culture characteristics: Colonies leathery, appressed, with fluffy aerial mycelium, somewhat folded at the middle, pale olivaceous grey to pale greenish grey after 2 wk in the dark at 25 °C. Colonies reaching 32–34 mm diam on MEA after 2 wk in the dark at 25 °C.
Materials examined: Philippines, on living leaf of unidentified palm, 2009, K.D. Hyde, culture CPC 20476. Thailand, Chiang Rai, on living leaves of unknown plant, 1 Jan. 2009, P.W. Crous, culture CPC 17762; Chiang Rai, house of K.D. Hyde, living leaves of Malus sp., 2009, P.W. Crous, culture CPC 21379.
Phragmocapnias plumeriae (Hongsanan & K.D. Hyde) Abdollahz. & Crous, comb. nov. MycoBank MB833164.
Basionym: Conidiocarpus plumeriae Hongsanan & K.D. Hyde, Mycosphere 6: 820. 2015.
Description: Hongsanan et al. (2015b).
Notes: This species was recently described by Hongsanan et al. (2015b) based on its sexual morph. Phylogenetically, it is closely related to P. betle (Figs S1, 2).
Polychaeton (Pers.) Lév., In: Orbigny, Dict. Univ. Hist. Nat. 8: 493. 1846.
Mycelium superficial or immersed, hyaline to brown, branched, consisting of smooth, thin-walled, septate hyphae, constricted at septa, with a mucilaginous outer wall layer. Conidiomata pycnidial, supported on a black mycelial network, narrow and cylindrical stalks composed of tightly compacted, anastomosed, synnematous cylindrical hyphae, lageniform with a brown oval or ellipsoid part which produces conidia and a long neck. The pycnidium wall is composed of two or more layers, the outer one being more or less pseudoparenchymatous although the short cells tend to be arranged linearly, indicating their origin from longitudinally fused hypha. Ostiole surrounded by hyaline, subulate, hyphal extensions. Conidia small, oblong to ellipsoid, continuous, aseptate, hyaline, smooth-walled, arranged in a droplet at the apex of pycnidium.
Type species: Polychaeton quercinum (Pers.) Kuntze
Notes: Polychaeton was introduced by Persoon as a sub-genus in Fumago, and raised to generic rank by Léveillé (1847). The taxonomy of Polychaeton was discussed by Hughes (1976) and Chomnunti et al. (2011). Hughes (1976) considered Po. citri and Po. quercinum suitable as generic types, and designated Po. quercinum as lectotype species of Polychaeton. Chomnunti et al. (2011) regarded Capnodium as sexual morph of Polychaeton, and chose Capnodium following the one fungus = one name concept. A search of Index Fungorum and MycoBank (March 2019) revealed several names in Polychaeton including Po. citri. In this study, we examined isolate CBS 116435 deposited in CBS as Po. citri (Pers.) Lév. from Iran on Citrus aurantium, isolated by Walter Gams. In the phylogenetic analyses (Figs S1, 2) this isolate clustered in a distinct clade close to Conidiocarpus and Phragmocapnias. Hughes (1976) mentioned that in Polychaeton pycnidia are supported on a stalk, have an ellipsoidal pycnidial cavity, with no conspicuous swelling, and terminate in a neck with hyaline hyphal extensions. On SNA isolate CBS 116435 produced pycnidia with a swollen body, a long neck, and a short stalk. On OA pycnidia tended to have much longer necks with no conspicuous swelling, while in Conidiocarpus pycnidia have a conspicuous swelling, a long neck, and are supported on a long stalk on both OA and SNA (Fig. 14). Therefore, we designate this clade as Polychaeton, although further studies are required to resolve the taxonomy of the various species described in the genus.
Neoantennariellaceae Abdollahz. & Crous, fam. nov. MycoBank MB833165.
Etymology: Name refers to the genus Neoantennariella.
Mycelium superficial or immersed, pale brown to brown, branched, consisting of smooth, thin-walled, septate hyphae, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial or immersed, mostly globose or cylindrical, pale to dark brown, intercalary, lateral or terminal on erect hyphal branches. Ostiole absent, or not well-developed, mostly releasing conidia by means of irregular rupture. Conidia hyaline, aseptate, ellipsoid to ovoid, with minute guttules, smooth, thin-walled.
Type genus: Neoantennariella Abdollahz. & Crous
Fumiglobus D.R. Reynolds & G.S. Gilbert, Cryptog. Mycol. 27: 252. 2006.
Type species: F. ficinus (Bat. et al.) D.R. Reynolds & G.S. Gilbert
Description: Reynolds & Gilbert (2006).
Note: According to Index Fungorum and MycoBank (March 2019), Fumiglobus presently contains 10 names, of which LSU and ITS sequences data are only available for F. pieridicola.
Neoantennariella Abdollahz. & Crous, gen. nov. MycoBank MB833166.
Etymology: Name reflects its morphological similarity to the genus Antennariella.
Mycelium superficial or immersed, pale brown to brown, branched, consisting of smooth, thin-walled, septate hyphae, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial or immersed, mostly globose or cylindrical, pale to dark brown, mostly intercalary, lateral or terminal on erect hyphal branches. Ostiole absent, or not well-developed, mostly releasing conidia by means of irregular rupture. Conidia hyaline, aseptate, ellipsoid to ovoid, with minute guttules, smooth, thin-walled.
Type species: Neoantennariella phylicae Abdollahz. & Crous
Note: Morphologically similar to Antennariella (see Hughes 1976, fig. 11), but different in conidiomatal and conidium morphology.
Neoantennariella phylicae Abdollahz. & Crous, sp. nov. MycoBank MB833167. Fig. 17.
Fig. 17.
Neoantennariella phylicae. A, B. Colony (2-wk-old) on MEA. C. Septate hyphae and immature conidioma on SNA. D–F. Intercalary, lateral and terminal conidiomata. G. Conidia. Scale bars: C–D = 20 μm; E–F = 25 μm; G = 10 μm.
Etymology: Name reflects the host genus Phylica from which it was isolated.
Mycelium superficial or immersed, pale brown to brown, branched, consisting of smooth, thin-walled, septate hyphae, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial or immersed, mostly globose or cylindrical, pale to dark brown, mostly intercalary, lateral or terminal on erect hyphal branches, meristogenous in development, pseudoparenchymatous, thin-walled, 1–2 cell layers of textura angularis, (40–)50–65(–85) × (30–)50–60(–68) μm (av. = 45 × 42 μm, n = 20). Ostiole absent, or not well-developed, mostly releasing conidia by means of irregular rupture. Conidia hyaline, aseptate, ellipsoid to ovoid, with minute guttules, smooth, thin-walled, (3.9–)4.5–5.2(–5.9) × 2.5–3.2(–3.6) μm (av. = 4.8 × 2.9 μm, n = 50).
Culture characteristics: Colonies leathery, appressed, with fluffy aerial mycelium; surface folded, edge sinuate, smoke grey to glaucous grey after 2 wk in the dark at 25 °C. Colonies reaching 17 mm diam on MEA after 2 wk in the dark at 25 °C.
Typus: UK, Inaccessible Island, on Phylica arborea, 30 Sep. 2011, P. Ryan (holotype CBS H-24298, culture ex-type CPC 19989 = CBS 146163).
Additional materials examined: South Africa, Marion Island, Prince Edward Is., on P. arborea, 2011, M.J. Wingfield, culture CPC 19227 = CBS 146164. UK, Inaccessible Island, on P. arborea, 30 Sep. 2011, P. Ryan, cultures CPC 19977 = CBS 146165, CPC 19981 = CBS 146166, CPC 19985 = CBS 146167, CPC 19992.
Neoasbolisia Abdollahz. & Crous, gen. nov. MycoBank MB833168.
Etymology: Name reflects its morphological similarity to the genus Asbolisia Bat. & Cif.
Mycelium superficial or immersed, pale brown to brown, branched, consisting of smooth, thin-walled, septate hyphae, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial or immersed, mostly globose or pyriform, brown to dark brown, intercalary, meristogenous in development, pseudoparenchymatous, thin-walled. Ostiole absent or not well-developed, mostly releasing conidia by means of irregular rupture. Conidia hyaline, aseptate, oblong to ellipsoid, with minute guttules, smooth, thin-walled.
Type species: Neoasbolisia phylicae Abdollahz. & Crous
Note: Morphologically similar to Asbolisia Bat. & Cif. (Nom. illegit., Art. 53.1), but as the latter is illegitimate, a new genus is introduced.
Neoasbolisia phylicae Abdollahz. & Crous, sp. nov. MycoBank MB833169. Fig. 18.
Fig. 18.
Neoasbolisia phylicae. A, B. Colony (2-wk-old) on MEA. C–E. Conidiomata on SNA. F. Conidia. Scale bars: C = 20 μm; D–E = 25 μm; F = 10 μm.
Etymology: Name reflects the host genus Phylica from which it was isolated.
Mycelium superficial or immersed, pale brown to brown, branched, consisting of smooth, thin-walled, septate hyphae, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial or immersed, mostly globose or pyriform, brown to dark brown, intercalary, meristogenous in development, pseudoparenchymatous, thin-walled, 1–2 cell layers of textura angularis, (63–)80–140(–180) × (56–)70–100(–148) μm (av. = 110 × 90 μm, n = 20). Ostiole absent or not well-developed, mostly releasing conidia by means of irregular rupture. Conidia hyaline, aseptate, oblong to ellipsoid, with minute guttules, smooth, thin-walled, (4.5–)4.6–5.2(–5.9) × (1.6–)1.8–2(–2.3) μm (av. = 5.2 × 2 μm, n = 50).
Culture characteristics: Colonies leathery, appressed, with fluffy aerial mycelium, edge sinuate, glaucous grey to olivaceous grey after 2 wk in the dark at 25 °C. Colonies reaching 14 mm diam on MEA after 2 wk in the dark at 25 °C.
Typus: UK, Inaccessible Island, on Phylica arborea, 30 Sep. 2011, P. Ryan (holotype CBS H-24299, culture ex-type CPC 19982 = CBS 146168).
Readerielliopsidaceae Abdollahz. & Crous, fam. nov. MycoBank MB833170.
Etymology: Name refers to the genus Readerielliopsis.
Mycelium superficial or immersed, hyaline to pale brown, branched, hyphae smooth, thin-walled, septate, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial, globose to pyriform, cylindrical to flask-shaped, short to long, straight to irregular, occurring singly or in groups, medium to dark brown, if synnematous, then with a hyaline to pale brown stalk forming a long neck. Ostiole absent, or present, with or without hyphal hairs. Conidia small, hyaline, smooth, aseptate, oblong to ellipsoid or obdeltoid, or pale to medium brown, transversely euseptate, filiform, fusoid-ellipsoidal.
Type genus: Readerielliopsis Crous & Decock
Fumagospora G. Arnaud, Ann. École Nat. Agric. Montpellier, Sér. 2 10: 326. 1911.
Type species: Fumagospora capnodioides G. Arnaud
As discussed by Hughes (1976) Fumagospora with hyaline and continuous conidia that become brown and transversely septate with one or more longitudinally septate cells, is the asexual morph of some Capnodium species (e.g. Capnodium salicinum). In this study Ca. salicinum (CBS 131.34) constitutes a distinct clade in Readerielliopsidaceae separate from other Capnodium species that grouped in Capnodium sensu Chomnunti et al. (2011). In a study on capnodiaceous sooty molds in Iran, phylogenetic analysis based on ITS sequence data placed isolate GUM 1315 with Fumagospora morphology (fig. 2, Khodaparast et al. 2020) close to Ca. salicinum CBS 131.34. Therefore, we designated Fumagospora as a generic name for this clade.
Phaeoxyphiella Bat. & Cif., Quad. Lab. Crittogam., Pavia 31: 145. 1963.
Type species: Ph. morototoni Bat. & Cif.
Notes: A search in Index Fungorum and MycoBank (March 2019) revealed seven names in Phaeoxyphiella. As discussed by Hughes (1976), Batista & Ciferri proposed this generic name for seven species, which fall into two groups based on conidial morphology. Four species, Ph. bahiensis, Ph. fischeri, Ph. morototoni and Ph. walteri, have long, fusoid or spindle-shaped, deeply pigmented, multiseptate phragmoconidia. Two species, Ph. californica and Ph. rondeletiae, have much shorter, oblong to ellipsoidal, 3-septate phragmoconidia, which are at first hyaline, slowly becoming brown. The seventh species, Ph. callitris, has 5-septate conidia, but is a nomen nudum because it lacks a Latin diagnosis. There are no cultures or sequences available for any of these species. In this study, we examined five isolates which based on phylogenetic inference clustered in two distinct clades (Figs S1, 2).
Phaeoxyphiella australiana Abdollahz. & Crous, sp. nov. MycoBank MB833171.
Etymology: Name reflects the country where it was collected, Australia.
Culture sterile. Phaeoxyphiella australiana, differs from its closest phylogenetic neighbour, Ph. phylicae, by unique allelles in four loci based on alignments of the separate loci deposited in TreeBASE as Study S25414: RPB2 positions 52(G), 55(G), 70(A), 91(C), 112(A), 136(G), 151(C), 178(A), 238(G), 259(G), 268(T), 298(T), 334(C), 346(A), 349(T), 367(T), 379(C), 382(T), 386(C), 388(G), 520(C), 529(T), 532(A), 535(C), 551(C), 586(C), 595(C), 607(T), 661(A), 667(C), 736(G), 805(A), 826(C), 862(C), 881(A), 882(T), 899(C), 904(C), 907(A), 937(C), 979(T), 988(A), 994(C), 1007(C), 1009(A), 1027(T), 1042(C), 1054(C), 1075(T); TEF-1α positions 60(C), 282(T), 283(T), 450(C), 489(T), 578(C), 596(C), 662(T), 674(G), 755(A), 788(T), 824(T), 848(C), 1011(A); ITS positions 67(C), 81(C), 149(T), 164(C), 412(A); LSU positions 414(T), 415(C), 609(C).
Typus: Australia, Western Australia, Denmark, Mount Lindesay Walk Trail, on Agonis sp., 19 Sep. 2015, P.W. Crous (holotype CBS H-24258, culture ex-type CPC 29527 = CBS 146169).
Note: Phaeoxyphiella australiana differs phylogenetically from Ph. phylicae in 3, 5, 14 and 50 bp in the LSU, ITS, TEF-1α and RPB2 sequences.
Phaeoxyphiella phylicae Abdollahz. & Crous, sp. nov. MycoBank MB833172. Fig. 19.
Fig. 19.
Phaeoxyphiella phylicae. A, B. Colony (2-wk-old) on MEA. C, D. Conidiomata with conidia on SNA. E. Transversely euseptate brown conidia. F, G. Spermogonia. H. Microconidia. Scale bars: C, E, F = 20 μm; D = 50 μm; G = 25 μm; H = 10 μm.
Etymology: Name reflects Phylica, the host genus from which it was collected.
Mycelium superficial or immersed, hyaline to medium brown, branched, hyphae smooth to slightly verruculose, thin-walled, septate, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial or immersed, glabrous, sessile, obclavate, pyriform or conoidal, brown, membraneous, thin-walled, 105–200 × 60–130 μm. Ostiole present, simple, without hyphal extensions. Conidia pale to medium brown, transversely euseptate, not constricted at septa, 11–19-celled, filiform, fusoid-ellipsoidal, straight to somewhat curved, ends rounded, often with a truncate base, smooth, (43–)67–80(–90) × (5.9–)6–9(–9.8) μm, (av. = 75 × 7.7 μm, n = 50). Spermatogonia superficial or immersed, globose to subglobose, pale to dark brown, mostly intercalary, lateral or terminal on erect hyphal branches, 49–69 × 43–62 μm. Ostiole absent, releasing microconidia by means of irregular rupture. Microconidia hyaline, aseptate, ellipsoid to ovoid, continuous, with minute guttules, smooth, thin-walled, (3.6–)4.2–4.9(–5.1) × (2–)2.2–2.7(–2.9) μm, (av. = 4.5 × 2.4 μm, n = 50).
Culture characteristics: Colonies, leathery, appressed, with fluffy aerial mycelium, folded, edge sinuate, glaucous grey to pale olivaceous grey after 2 wk in the dark at 25 °C. Colonies reaching 18 mm diam on MEA after 2 wk in the dark at 25 °C.
Typus: UK, Inaccessible Island, on Phylica arborea, 30 Sep. 2011, P. Ryan (holotype CBS H-24300, culture ex-type CPC 19993 = CBS 146170).
Additional material examined: UK, Inaccessible Island, on P. arborea, 30 Sep. 2011, P. Ryan cultures CPC 19979 = CBS 146171, CBS H-24259, CPC 19984 = CBS 146172, CPC 19987 = CBS 146173, CBS H-24260.
Notes: Phaeoxyphiella phylicae differs from all seven species described by Batista & Ciferri (1963a) by producing 11–19-celled phragmoconidia. Conidial dimensions in Ph. phylicae are close to those of Ph. walteri, but conidia are 11–19-celled in Ph. phylicae, and 3–15-celled in Ph. walteri.
Readerielliopsis Crous & Decock, Persoonia 34: 195. 2015.
Type species: Readerielliopsis fuscoporiae Crous & Decock
Description: See Crous et al. (2015).
Note: Readerielliopsis includes two species, R. fuscoporiae (isolated from basidiomata of Fuscoporia wahlbergii) and R. guyanensis (isolated from the decaying leaf of an angiosperm).
Scolecoxyphium Cif. & Bat., Publicações Inst. Micol. Recife 47: 5. 1956.
Type species: Scolecoxyphium fraserae Cif. & Bat.
Mycelium superficial or immersed, hyaline to brown, branched, hyphae smooth, thin-walled, septate, constricted at septa, with a mucilaginous outer wall layer. Pycnidia irregularly cylindrical, straight or flexuous, short or long, sessile, with no swollen part to indicate the location of the pycnidial cavity; wall is composed of linearly arranged, fused hyphae. Ostiole present, without hyaline hyphal extensions; around the ostiole the hyphae are brown and obtusely rounded. Conidia ellipsoidal, hyaline and continuous (adapted from Hughes 1976).
Notes: A search in Index Fungorum and MycoBank (March 2019) revealed four names in Scolecoxyphium. This genus was established by Ciferri and Batista based on the type species, S. fraserae (Ciferri et al. 1956). Three additional species were introduced by Batista & Ciferri (1963a). No cultures or sequence data exist for any of these species. In the phylogenies generated here, a highly supported clade was resolved resembling Scolecoxyphium (Figs S1, 2). The taxa studied here differed from all previously described species of Scolecoxyphium, and are therefore described as new.
Scolecoxyphium blechni Abdollahz. & Crous, sp. nov. MycoBank MB833173. Fig. 20.
Fig. 20.
Scolecoxyphium blechni. A, B. Colony (2-wk-old) on MEA. C–H. Irregularly cylindrical-oblong, straight or flexuous, simple or branched conidiomata on SNA. I. Conidia. Scale bars: C = 50 μm; D, F–H = 20 μm; E = 25 μm; I = 10 μm.
Etymology: Name reflects the host genus Blechnum from which it was isolated.
Mycelium superficial or immersed, hyaline to brown, branched, hyphae smooth, thin-walled, septate, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial or immersed, irregularly cylindrical-oblong, straight or flexuous, long, simple or branched, occurring singly or in groups, medium to dark brown, synnematous, on SNA 80–225 × 18–40 μm (av. = 160 × 24 μm, n = 20), on OA 180–420 × 30–57 μm (av. = 225 × 38 μm, n = 20), sessile, without swollen part and neck; wall comprising mostly of cylindrical cells. Ostiole at apex of pycnidia, without hyaline hyphal extensions. Conidia small, hyaline, aseptate, oblong to ellipsoid, continuous, (3.3–)3.5–4(–4.4) × 1.3–1.7 μm (av. = 3.8 × 1.5 μm, n = 50).
Culture characteristics: Colonies, leathery, appressed, with fluffy aerial mycelium, folded, edge sinuate, glaucous grey to pale olivaceous grey after 2 wk in the dark at 25 °C. Colonies reaching 11 mm diam on MEA after 2 wk in the dark at 25 °C.
Typus: UK, Inaccessible Island, on Blechnum palmiforme, 30 Sep. 2011, P. Ryan (holotype CBS H-24301, culture ex-type CPC 19990 = CBS 146174).
Notes: Phylogenetically (Figs S1, 2) S. blechni is closely related to S. blechnicola. Morphologically S. blechni resembles S. leucadendri, but pycnidia of S. leucadendri (av. = 500 × 30 μm) on OA are much longer than those of S. blechni (av. = 225 × 38 μm). Moreover, these two species differ in their geographical origin (S. blechni from UK, S. leucadendri from South Africa) and substrate (S. blechni on Blechnum palmiforme, S. leucadendri on Leucadendron sp.).
Scolecoxyphium blechnicola Abdollahz. & Crous, sp. nov. MycoBank MB833174.
Etymology: Name reflects the host genus Blechnum from which it was isolated.
Culture sterile. Scolecoxyphium blechnicola differs from its closely related species, Scolecoxyphium blechni by unique alleles in four loci based on alignments of the separate loci deposited in TreeBASE as Study S25414: RPB2 positions 58(T), 82(T), 133(G), 355(T), 397(T), 403(C), 472(C), 475(C), 481(A), 551(T), 577(G), 580(A), 664(G), 667(C), 985(G), 994(C); TEF-1α positions 447(C), 683(G), 737(T), 761(C), 803(T), 878(T), 969(T), 1023(C); ITS positions 92(T), 93(C), 180(C), 515(T).
Typus: UK, Inaccessible Island, on Blechnum palmiforme, 30 Sep. 2011, P. Ryan (holotype CBS H-24261, culture ex-type CPC 19991 = CBS 146175).
Note: S. blechnicola differs from S. blechni by 4, 8 and 17 nucleotides in ITS, TEF-1α and RPB2 loci, respectively.
Scolecoxyphium leucadendri Abdollahz. & Crous, sp. nov. MycoBank MB833175. Fig. 21.
Fig. 21.
Scolecoxyphium leucadendri. A, B. Colony (2-wk-old) on MEA. C. Immature conidiomata. D–F. Irregularly cylindrical-oblong, straight or flexuous, simple or branched conidiomata on SNA. G. Conidia inside conidiomata. H. Conidia. Scale bars: C, E = 20 μm; D, F = 25 μm; G, H = 10 μm.
Etymology: Name reflects the host genus Leucadendron from which it was isolated.
Mycelium superficial or immersed, hyaline to brown, branched, consisting of smooth, thin-walled, septate hyphae, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial or immersed, irregularly cylindrical-oblong, straight or flexuous, long, simple or branched, occurring singly or in groups, medium to dark brown, synnematous; on SNA 104–178 × 20–37 μm (av. = 150 × 25 μm, n = 20), on OA 350–700 × 18–39 μm (av. = 500 × 30 μm, n = 20), sessile, without swelling part and neck, wall comprising mostly cylindrical cells. Ostiole at apex of pycnidia, without hyaline hyphal extensions. Conidia small, hyaline, aseptate, oblong to ellipsoid, continuous, 3–4 × 1.3–1.6 μm (av. = 3.5 × 1.5 μm, n = 50).
Culture characteristics: Colonies, leathery, appressed, with fluffy aerial mycelium, folded, edge sinuate, smoke grey to olivaceous grey after 2 wk in the dark at 25 °C. Colonies reaching 12 mm diam on MEA after 2 wk in the dark at 25 °C.
Typus: South Africa, Western Cape Province, Hermanus, Fernkloof, on leaves of Leucadendron sp., 2 May 2010, P.W. Crous (holotype CBS H-24302, culture ex-type CPC 18313 = CBS 146176).
Notes: Scolecoxyphium leucadendri formed a distinct clade in the phylogenetic trees (Figs S1, 2), but is morphologically similar to S. blechni. The two species can be differentiated based on the pycnidial size on OA, geography and substrate.
Scolecoxyphium phylicae Abdollahz. & Crous, sp. nov. MycoBank MB833176. Fig. 22.
Fig. 22.
Scolecoxyphium phylicae. A, B. Colony (2-wk-old) on MEA. C–F. Cylindrical-oblong, straight, simple conidiomata on SNA. G. Conidia. Scale bars: C = 25 μm; D–F = 20 μm; G = 10 μm.
Etymology: Name reflects the host genus Phylica from which it was isolated.
Mycelium superficial or immersed, hyaline to brown, branched, hyphae smooth, thin-walled, septate, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial or immersed, cylindrical-oblong, straight, short, simple, occur singly or in groups, medium to dark brown, synnematous, 40–65 × 27–37 μm (av. = 52 × 32 μm, n = 20), sessile, without swollen part and neck, wall comprising mostly cylindrical cells. Ostiole at apex of pycnidia, surrounded with brown and obtusely rounded hyphae, without hyaline hyphal extensions. Conidia small, hyaline, aseptate, oblong to ellipsoid, continuous, (3.6–)4–4.3(–4.7) × 1.2–1.7 μm (av. = 4.2 × 1.4 μm, n = 50).
Culture characteristics: Colonies, leathery, appressed, with fluffy aerial mycelium, folded, edge sinuate, smoke grey to olivaceous grey with a pink pigment after 2 wk in the dark at 25 °C. Colonies reaching 15 mm diam on MEA after 2 wk in the dark at 25 °C.
Typus: South Africa, Marion Island, Prince Edward Is., on Phylica arborea, 2011, M.J. Wingfield (holotype CBS H-24303, culture ex-type CPC 19219 = CBS 146177).
Additional material examined: South Africa, Marion Island, Prince Edward Is., on P. arborea, 2011, M.J. Wingfield, culture CPC 19225 = CBS 146178.
Notes: Genetically S. phylicae constitutes a separate clade within Scolecoxyphium (Figs S1, 2). It is morphologically distinguishable based on its short cylindrical pycnidia and longer conidia.
Scorias Fr., Syst. Mycol. (Lunde) 3(2): 269. 1832.
Type species: Scorias spongiosa (Schwein.) Fr.
Notes: A search in Index Fungorum and MycoBank (March 2019) revealed 13 names in Scorias. Chomnunti et al. (2011) re-examined the type species, Sc. spongiosa, and designated an epitype MFLU10-0013 (ex-epitype MFLUCC10-0084) from Thailand. Cultures and molecular data are presently available for three species: Sc. leucadendri (LSU, ITS, TEF-1α and RPB2), Sc. mangiferae (LSU and ITS) and Sc. spongiosa (LSU). In this study we examined and sequenced isolates CBS 201.30 and CBS 325.33, which are representatives of a new species described here.
Scorias aphidis Abdollahz. & Crous, sp. nov. MycoBank MB833177. Fig. 23.
Fig. 23.
Scorias aphidis. A, B. Colony (2-wk-old) on MEA. C, D. Flask-shape conidiomata on SNA. E. Ostioles surround by hyaline hyphae. F, G. Conidia produced in ellipsoidal part of conidiomata. H. Conidia. Scale bars: D = 50 μm; E–F = 25 μm; G–H = 10 μm.
Etymology: Name reflects the fact that it was isolated from an aphid.
Mycelium superficial or immersed, hyaline to pale brown, branched, hyphae smooth, thin-walled, septate, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial, flask-shape, simple, occurring singly or in groups, medium to dark brown, synnematous, 200–505 μm high (av. = 395 μm, n = 20), with a hyaline to pale brown stalk (50–108 × 32–74 μm, av. = 80 × 50 μm), conspicuous oval or ellipsoidal central part (90–260 × 42–135 μm, av. = 210 × 80 μm), and a long neck (50–185 × 15–60 μm, av. = 115 × 20 μm); wall comprising mostly cylindrical cells. Ostiole at pycnidial apex, surrounded by hyaline hyphae. Conidia small, hyaline, aseptate, oblong to ellipsoid, (4.9–)5.5–6.5(–7.6) × (1.8–)2–2.6 μm (av. = 6.2 × 2.2 μm, n = 50).
Culture characteristics: Colonies leathery, appressed, with fluffy aerial mycelium and creamy exudates conidia; surface folded, edge sinuate, glaucous grey to olivaceous grey after 2 wk in the dark at 25 °C. Colonies reaching 18 mm diam on MEA after 2 wk in the dark at 25 °C.
Typus: Country unknown, on aphid, 1933, dep. L.H. Leonian (holotype CBS H-24272, culture ex-type CBS 325.33).
Notes: Phylogenetically (Figs S1, 2) Sc. aphidis clustered in a clade containing Sc. mangiferae, Sc. spongiosa and Sc. camelliae, but differs from Sc. mangiferae in 8 bp in LSU and 7 bp in ITS, from Sc. mangifera in 7 bp in LSU and from Sc. camelliae in 9 bp in LSU, 8 bp in ITS, 28 bp in TEF-1α and 94 bp in RPB2. Morphologically, conidia of Sc. aphidis (av. = 6.2 × 2.2 μm, on SNA) are larger than those of Sc. spongiosa (av. = 3.9 × 1.9 μm on PDA) and Sc. camelliae (av. = 5.6 × 2.4 μm on SNA), but similar to conidia of Sc. mangiferae (av. = 6.7 × 2.5 μm in vivo). Moreover, pycnidia of Sc. camelliae (av. = 100 μm) are much shorter than those of Sc. aphidis (av. = 395 μm).
Scorias camelliae Abdollahz. & Crous, sp. nov. MycoBank MB833178. Fig. 24.
Fig. 24.
Scorias camelliae. A, B. Colony (2-wk-old) on MEA. C. Flask-shape conidiomata on SNA. D. Ostiole surround by hyaline hyphae. E. Conidia. Scale bars: C–D = 20 μm; E = 10 μm.
Etymology: Name reflects the host genus Camillia from which it was collected.
Mycelium superficial or immersed, hyaline to pale brown, branched, hyphae smooth, thin-walled, septate, constricted at septa, with a mucilaginous outer wall layer. Pycnidia superficial, flask-shape, simple, occurring singly or in groups, medium to dark brown, synnematous, 59–163 μm high (av. = 100 μm, n = 20), sessile or with short stalk (11–30 × 12–24 μm, av. = 15 × 17 μm), with conspicuous oval or ellipsoidal central part (37–85 × 16–57 μm, av. = 45 × 35 μm), and a long neck (19–77 × 6–27 μm, av. = 45 × 12 μm); wall comprising mostly cylindrical cells. Ostiole at apex of pycnidia, surrounded by hyaline hyphae. Conidia small, hyaline, aseptate, ellipsoid to ovoid, (4.4–)5.3–5.7(–7.4) × (1.9–)2.2–2.5(–2.9) μm (av. = 5.6 × 2.4 μm, n = 50).
Culture characteristics: Colonies, leathery, appressed, with fluffy aerial mycelium; surface folded, edge sinuate, dirty white to olivaceous grey after 2 wk in the dark at 25 °C. Colonies reaching 20 mm diam on MEA after 2 wk in the dark at 25 °C.
Typus: Indonesia, Java island, Bogor, Buitenzorg, on Camellia sinensis leaves, 1930, isol. Steinmann, dep. F.H. van Beyma (holotype CBS H-24273, culture ex-type CBS 201.30).
Notes: In the phylogenetic analyses (Fig. 2), Sc. camelliae clustered with Sc. spongiosa and Sc. mangiferae, but differs from Sc. spongiosa in 8 bp in LSU and from Sc. mangiferae in 10 bp in LSU and 1 bp in ITS. Morphologically, conidia of Sc. camelliae (av. = 5.6 × 2.4 μm on SNA) are larger than those of Sc. spongiosa (av. = 3.9 × 1.9 μm on PDA) and smaller than conidia of Sc. mangiferae (av. = 6.7 × 2.5 μm in vivo).
Discussion
The Capnodiales was originally established for three families of sooty moulds, namely Antennulariaceae, Capnodiaceae and Coccodiniaceae (Woronichin 1925). Schoch et al. (2006) transferred Mycosphaerellaceae and Piedraiaceae to the Capnodiales and recognised the Cladosporiaceae (= Davidiellaceae), thereby expanding the concept of the order. Subsequent phylogenetic studies further expanded the concept of Capnodiales, making it the second largest order of Dothideomycetes. These included fungi with a broad spectrum of morphology, life-styles and modes of nutrition, accommodating saprobes, plant and human pathogens, mycoparasites, lichenised and rock-inhabiting fungi, including epi-, ecto- and endophytes (Crous et al., 2009, Schoch et al., 2006, Schoch et al., 2009, Schoch and Grube, 2015). Based on this broad definition, we have included a collection of isolates representing 11 families: Capnodiaceae, Cladosporiaceae, Cystocoleaceae, Dissoconiaceae, Extremaceae, Mycosphaerellaceae, Neodevriesiaceae, Phaeothecaceae, Phaeothecoidiellaceae, Racodiaceae, Schizothyriaceae, Teratosphaeriaceae (including Piedraiaceae) and two incertae sedis genera, Comminutispora and Phaeotheca.
Previous phylogenetic studies on Capnodiales all suffered from a limited sampling of true sooty moulds, an aspect that we have addressed in the present study. In addition, we have included two protein-coding genes (TEF-1α and RPB2) together with rDNA sequence data (LSU and ITS) to achieve a more stable and robustly supported phylogeny for this extremely diverse group of fungi.
Sooty moulds are presently classified in seven families, with some miscellaneous genera in either Dothideomycetes or Eurotiomycetes (Chomnunti et al. 2014). Dothideomycetous sooty moulds belong to four families, namely Antennulariellaceae, Capnodiaceae, Euantennariaceae and Metacapnodiaceae. Although many studies have focused on the taxonomy of sooty moulds (Hughes, 1951, Hughes, 1966, Hughes, 1972, Hughes, 1976, Hughes, 1981, Hughes, 2003, Hughes, 2007, Yamamoto, 1954, Batista and Ciferri, 1963a, Batista and Ciferri, 1963b, Reynolds, 1975, Reynolds, 1979, Reynolds, 1986, Reynolds, 1998, Faull et al., 2002, Reynolds and Gilbert, 2005, Reynolds and Gilbert, 2006, Crous et al., 2007b, Crous et al., 2011a, Crous et al., 2011b, Ruíz et al., 2008, Cheewangkoon et al., 2009, Chomnunti et al., 2011, Chomnunti et al., 2014, Ren et al., 2012, Bose et al., 2014, Yang et al., 2014, Hongsanan et al., 2015a, Hongsanan et al., 2015b, Liu et al., 2015), cultures for inclusion in the present study were available only for Capnodiaceae.
In an attempt to explain the high levels of diversity in the Capnodiales, the resulting phylogenetic tree (LSU, TEF-1α and RPB2) revealed Capnodiales s. lat. as polyphyletic, representing seven orders. As a result, Capnodiales s. str. was redefined, the Mycosphaerellales was resurrected, and five new orders were introduced. These include the Cladosporiales, Comminutisporales, Neophaeothecales, Phaeothecales and the Racodiales.
All sooty mould isolates in both the RAxML and Bayesian analyses, constituted a well-supported monophyletic clade thus defining Capnodiales s. str. (ML-BS = 90 %, PP = 1). The monophyly of the sooty moulds was also supported by their unique morphology, ecology and mode of nutrition. Sooty moulds are epiphytes usually with dark-coloured and mucilaginous hyphae, occurring superficially on living plants. They are often associated with insects producing honeydew, and derive nutrients from the excretion of these insects, but they can occur also without insects and absorb other nutrients (Hughes, 1976, Crous et al., 2009, Chomnunti et al., 2014). In the present study three families are recognised in Capnodiales s. str., namely Capnodiaceae, Neoantennariellaceae and Readerielliopsidaceae of which the latter two are newly described. Seven genera are recognised in Capnodiaceae: Capnodium, Cheatocapnodium, Conidiocarpus, Heterconium, Leptoxyphium, Phragmocapnias and Polychaeton. Based on morphology and phylogenetic analyses Phragmocapnias has been resurrected and a further 11 new species have been introduced in the family, which includes both hyphomycetous and coelomycetous asexual morphs.
The Neoantennariellaceae is introduced to accommodate Fumiglobus and two new monotypic genera, Neoantennariella and Neoasbolisia. All three genera produce pycnidial conidiomata. In Readerielliopsidaceae four coelomycetous genera, namely Phaeoxyphiella, Readerielliopsis, Scolecoxyphium and Scorias, and eight new species have been recognised.
Hawksworth et al. (1995) introduced Mycosphaerellaceae in the Dothideales, while Kirk et al. (2001) elevated this family to the order Mycosphaerellales, and Schoch et al. (2006) and Kirk et al. (2008) again placed it as a family in the Capnodiales. Despite high support values obtained in subsequent phylogenetic studies (Crous et al., 2009, Schoch et al., 2009, Suetrong et al., 2009, Hyde et al., 2013), the Mycosphaerellales was never resurrected, and the Capnodiales was applied in a broad sense beyond the original concept presented by Woronichin (1926) to accommodate the sooty moulds. In our multigene phylogeny (LSU, TEF-1α and RPB2) using both RAxML and Bayesian analyses, we found Mycosphaerellales to represent a fully supported clade (ML-BS = 100 %, PP = 1) accommodating eight families, namely Cystocoleaceae, Dissoconiaceae, Extremaceae, Mycosphaerellaceae, Neodevriesiaceae, Phaeothecoidiellaceae, Schizothyriaceae and Teratosphaeriaceae. We have consequently resurrected Mycosphaerellales as a separate order and provided an amended description for it. Although the Mycosphaerellales includes species that are saprobes, ectophytes, plant pathogens and lichenised fungi, this order is mainly characterised by plant pathogenic fungi that are commonly isolated as endophytes, being ecologically distinct from the sooty moulds, which are epiphytes. Xu et al. (2017) and Ismail et al. (2016) showed that the ectophytic sooty blotch and flyspeck fungi (Dissoconiaceae, Phaeothecoidiellaceae, Schizothyriaceae) have evolved from ancestral phytopathogenic relatives. Lichenisation has occurred once in Cystocoleaceae, while plant pathogens are found in Mycosphaerellaceae, Neodevriesiaceae and Teratosphaeriaceae and human pathogens have evolved in Teratosphaeriaceae. To better elucidate the general evolutionary pattern, a greater number of samples and genome-wide comparative analyses will be conducted in future studies.
Members of Cladosporiaceae are chiefly saprobic and endophytic, with a few species that are fungicolous, lichenicolous, or plant pathogenic. In a series of phylogenetic studies, members of Cladosporiaceae were resolved as a distinct clade apart from Capnodiaceae, Mycosphaerellaceae and allied families: Dissoconiaceae, Extremaceae, Neodevriesiaceae, Phaeothecoidiellaceae, Schizothyriaceae and Teratosphaeriaceae (Crous et al., 2009, Schoch et al., 2006, Schoch et al., 2009, Suetrong et al., 2009, Bensch et al., 2012, Hyde et al., 2013, Van Nieuwenhuijzen et al., 2016, Videira et al., 2017). In the present study, Cladosporiaceae clustered apart from Mycosphaerellales and Capnodiales s. str. and formed a distinct clade sister to Comminutisopora agavaciensis. Therefore, Cladosporiaceae has been elevated to ordinal level as Cladosporiales. Morphologically, members of Cladosporiales are quite distinct from those of Mycosphaerellales, having long, solitary, flexuous conidiophores with chains of dry, pigmented conidia. Ecologically, conidia of Cladosporiales can rehydrate, germinate and grow within hours, while members of Mycosphaerellales are generally slow to reactivate, and far less hardy to extremes in temperature and moisture conditions.
Endoconidial taxa within Capnodiales s. lat. that belong to Comminutispora and Phaeotheca have received considerable attention (Sigler et al., 1981, Ramaley, 1996, de Hoog et al., 1997, de Hoog et al., 1999, Zalar et al., 1999, Crous et al., 2009, Crous et al., 2016, Crous et al., 2018). These saprobic fungi (de Hoog et al., 1999, Crous et al., 2009) were found to occupy a basal position in the phylogenetic tree (Figs S1, Fig. 1, Fig. 3), representing three new orders, namely Comminutisporales, Neophaeothecales and Phaeothecales. Furthermore, our results have resolved Phaeotheca as polyphyletic, representing two distinct clades. Phaeotheca fissurella (Phaeothecales ord. nov.) clustered apart from P. salicornia and P. triangularis in a clade together with the lichen Racodium rupestre. Racodium rupestre clusters in a separate clade apart from another lichen species, Cystocoleus ebeneus (Cystocoleaceae, Mycosphaerellales), and represents a new order, Racodiales ord. nov. Phaeotheca salicornia and P. triangularis occupied the basal position in the phylogenetic tree (Figs S1, Fig. 1, Fig. 3) as one of the earliest lineages in Capnodiales s. lat., for which we introduced a new genus Neophaeotheca (Neophaeothecaceae fam. nov. and Neophaeothecales ord. nov.). Members of Phaeothecales, Racodiales and Neophaeothecales are commonly isolated under more dry, extreme conditions.
The present study has provided a more stable backbone for the phylogeny of sooty moulds and allied taxa formerly classified in what was circumscribed as “Capnodiales s. lat.” Many families are not yet represented in our phylogenetic analysis, pending further collections. Although our results revealed Capnodiales s. lat. as polyphyletic, including seven different orders, this remains a work in progress. Furthermore, phylogenetic ancestral reconstruction analysis has revealed the saprobic lifestyle to be a primitive state in Capnodiales s. lat. (see Neophaeothecales; Fig. 3), while Haridas et al. (2020) also showed the ancestral state of Dothideomycetes to be saprobic. Several transitions have occurred to evolve lichenised, epiphytic and plant and human pathogenic lifestyles (Hongsanan et al. 2016, Ametrano et al. 2019), with the sooty mould ecology apparently having evolved more recently. A more robust sampling of the unexplored or little-known clades of Dothideomycetes, and genome-wide comparative analyses will provide greater clarity on the evolutionary patterns of lifestyles and modes of nutrition, that has made it possible for communities of Dothideomycetes to adapt to changing environmental conditions.
Acknowledgements
Jafar Abdollahzadeh was supported by the University of Kurdistan, Sanandaj, Kurdistan Province, Iran during his sabbatical leave at the Westerdijk Fungal Biodiversity Institute.
Footnotes
Peer review under responsibility of Westerdijk Fungal Biodiversity Institute.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.simyco.2020.02.004.
Contributor Information
J. Abdollahzadeh, Email: j.abdollahzadeh@uok.ac.ir.
P.W. Crous, Email: p.crous@wi.knaw.nl.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Original phylogenetic tree inferred from a Bayesian analysis based on a concatenated alignment of LSU, TEF-1α and RPB2. Bayesian posterior probabilities (BPP) and maximum likelihood bootstrap support values (MLBS) are indicated at the nodes (BPP/MLBS). The scale bar represents the expected number of changes per site. Families and orders are highlighted in blocks of different colour and indicated to the right of the tree. The tree was rooted with Venturia inaequalis (CBS 594.70).
References
- Ametrano C.G., Grewe F., Crous P.W., et al. Genome-scale data resolve ancestral rock-inhabiting lifestyle in Dothideomycetes (Ascomycota) IMA Fungus. 2019;10:19. doi: 10.1186/s43008-019-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aptroot A. CBS-KNAW Fungal Biodiversity Centre; Utrecht, The Netherlands: 2006. Mycosphaerella and its anamorphs 2. Conspectus of mycosphaerella; pp. 1–231. CBS Biodiversity Series 5. [Google Scholar]
- Batista A.C., Ciferri R. Capnodiales. Saccardoa. 1963;2:1–296. [Google Scholar]
- Batista A.C., Ciferri R. The sooty-molds of the family Asbolisiaceae. Quaderno del Laboratorio Crittogamico del Istituto Botanico dell'Università di Pavia. 1963;31:1–229. [Google Scholar]
- Bensch K., Braun U., Groenewald J.Z., et al. The genus Cladosporium. Studies in Mycology. 2012;72:1–401. doi: 10.3114/sim0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra J.D., Oliveira R.J., Paiva L.M., et al. Bezerromycetales and Wiesneriomycetales ord. nov. (class Dothideomycetes), with two novel genera to accommodate endophytic fungi from Brazilian cactus. Mycological Progress. 2017;16:297–309. [Google Scholar]
- Bose T., Reynolds D.R., Berbee M.L. Common, unsightly and until now undescribed: Fumiglobus pieridicola sp. nov., a sooty mold infesting Pieris japonica from western North America. Mycologia. 2014;106:746–756. doi: 10.3852/13-288. [DOI] [PubMed] [Google Scholar]
- Cheewangkoon R., Groenewald J.Z., Hyde K.D., et al. Chocolate spot disease of Eucalyptus. Mycological Progress. 2012;11:61–69. [Google Scholar]
- Cheewangkoon R., Groenewald J.Z., Summerell B.A., et al. Myrtaceae, a cache of fungal biodiversity. Persoonia. 2009;23:55–85. doi: 10.3767/003158509X474752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomnunti P., Hongsanan S., Aguirre-Hudson B., et al. The sooty moulds. Fungal Diversity. 2014;66:1–36. [Google Scholar]
- Chomnunti P., Schoch C.L., Aguirre-Hudson B., et al. Capnodiaceae. Fungal Diversity. 2011;51:103–134. doi: 10.1007/s13225-011-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciferri R., Batista A.C., Nascimento M.L. Two new genera of pycnidiaceous sooty-molds associated with Microxyphium and Septonema. Publicações do Instituto de Micologia da Universidade do Recife. 1956;47:1–7. [Google Scholar]
- Crous P.W., Braun U., Groenewald J.Z. Mycosphaerella is polyphyletic. Studies in Mycology. 2007;58:1–32. doi: 10.3114/sim.2007.58.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Gams W., Stalpers J.A., et al. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology. 2004;50:19–22. [Google Scholar]
- Crous P.W., Groenewald J.Z., Shivas R.G., et al. Fungal Planet description sheets: 69–91. Persoonia. 2011;26:108–156. doi: 10.3767/003158511X581723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Luangsa-Ard J.J., Wingfield M.J., et al. Fungal Planet description sheets: 785–867. Persoonia. 2018;41:238–417. doi: 10.3767/persoonia.2018.41.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Mohammed C., Glen M., et al. Eucalyptus microfungi known from culture. 3. Eucasphaeria and Sympoventuriagenera nova, and new species of Furcaspora, Harknessia, Heteroconium and Phacidiella. Fungal Diversity. 2007;25:19–36. [Google Scholar]
- Crous P.W., Schoch C.L., Hyde K.D., et al. Phylogenetic lineages in the Capnodiales. Studies in Mycology. 2009;64:17–47. doi: 10.3114/sim.2009.64.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Summerell B.A., Shivas R.G., et al. Fungal Planet description sheets: 92–106. Persoonia. 2011;27:130–162. doi: 10.3767/003158511X617561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Verkley G.J.M., Groenewald J.Z., et al. Westerdijk Fungal Biodiversity Institute; Utrecht, The Netherlands: 2019. Fungal biodiversity; pp. 1–425. Westerdijk Laboratory Manual Series 1. [Google Scholar]
- Crous P.W., Wingfield M.J., Cheewangkoon R., et al. Foliar pathogens of eucalypts. Studies in Mycology. 2019;94:125–298. doi: 10.1016/j.simyco.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Guarro J., et al. Fungal Planet description sheets: 320–370. Persoonia. 2015;34:167–266. doi: 10.3767/003158515X688433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Richardson D.M., et al. Fungal Planet description sheets: 400–468. Persoonia. 2016;36:316–458. doi: 10.3767/003158516X692185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoog G.S., Beguin H., Batenburg-Van de Vegte W.H. Phaeotheca triangularis, a new meristematic black yeast from a humidifier. Antonie van Leeuwenhoek. 1997;71:289–295. doi: 10.1023/a:1000156820793. [DOI] [PubMed] [Google Scholar]
- de Hoog GS de, Zalar P., Urzi C., et al. Relationships of dothideaceous black yeasts and meristematic fungi based on 5.8S and ITS2 rDNA sequence comparison. Studies in Mycology. 1999;43:31–37. [Google Scholar]
- Faull J.L., Olejnik I., Ingrouille M., et al. A reassessment of the taxonomy of some tropical sooty moulds. Tropical Mycology. 2002;2:33–40. [Google Scholar]
- Haridas S., Albert R., Binder M., et al. 101 Dothideomycetes genomes: a test case for predicting lifestyles and emergence of pathogens. Studies in Mycology. 2020 doi: 10.1016/j.simyco.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth D.L., Kirk P.M., Sutton B.C., et al. 8th edn. CAB International; Wallingford, UK: 1995. Ainsworth and Bisby’s Dictionary of the Fungi. [Google Scholar]
- Hawksworth D.L., Santesson R., Tibell L. Racoleus, a new genus of sterile filamentous lichen-forming fungi from the tropics, with observations on the nomenclature and typification of Cystocoleus and Racodium. IMA Fungus. 2011;2:71–79. doi: 10.5598/imafungus.2011.02.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongsanan S., Hyde K.D., Bahkali A.H., et al. Fungal biodiversity profiles 11–20. Cryptogamie Mycologie. 2015;36:355–381. [Google Scholar]
- Hongsanan S., Sánchez-Ramírez S., Crous P.W., et al. The evolution of fungal epiphytes. Mycosphere. 2016;7:1690–1712. [Google Scholar]
- Hongsanan S., Tian Q., Hyde K.D., et al. Two new species of sooty moulds, Capnodium coffeicola and Conidiocarpus plumeriae in Capnodiaceae. Mycosphere. 2015;6:814–824. [Google Scholar]
- Hongsanan S., Zhao R.L., Hyde K.D. A new species of Chaetothyrina on branches of mango, and introducing Phaeothecoidiellaceae fam. nov. Mycosphere. 2017;8:137–146. [Google Scholar]
- Huelsenbeck J.P., Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Hughes S.J. Studies on micro-fungi X. Zygosporium. Mycological Papers. 1951;44:1–18. [Google Scholar]
- Hughes S.J. New Zealand fungi 7. Capnocybe and Capnophialophora, new form genera of sooty moulds. New Zealand Journal of Botany. 1966;4:333–353. [Google Scholar]
- Hughes S.J. New Zealand fungi 17. Pleomorphism in Cuantennariaceae and Metacapnodiaceae, two new families of sooty moulds. New Zealand Journal of Botany. 1972;10:225–242. [Google Scholar]
- Hughes S.J. Sooty moulds. Mycologia. 1976;68:451–691. [Google Scholar]
- Hughes S.J. New Zealand fungi 31. Capnobotrys, an anamorph of Metacapnodiaceae. New Zealand Journal of Botany. 1981;19:193–226. [Google Scholar]
- Hughes S.J. Capnofrasera dendryphioides, a new genus and species of sooty moulds. New Zealand Journal of Botany. 2003;41:139–146. [Google Scholar]
- Hughes S.J. Heteroconium and Pirozynskiella n. gen., with comments on conidium transseptation. Mycologia. 2007;99:628–638. doi: 10.3852/mycologia.99.4.628. [DOI] [PubMed] [Google Scholar]
- Hyde K.D., Jones E.G., Liu J.K., et al. Families of Dothideomycetes. Fungal Diversity. 2013;63:1–313. [Google Scholar]
- Ismail S.I., Batzer J.C., Harrington T.C., et al. Ancestral state reconstruction infers phytopathogenic origins of sooty blotch and flyspeck fungi on apple. Mycologia. 2016;108:292–302. doi: 10.3852/15-036. [DOI] [PubMed] [Google Scholar]
- Jaczewski A.A. Publishing House of SL Kind; Petrograd: 1917. Key to Fungi. Vol. II. Fungi Imperfecti. (In Russian) [Google Scholar]
- Jaklitsch W.M., Fournier J., Dai D.Q., et al. Valsaria and the Valsariales. Fungal Diversity. 2015;73:159–202. doi: 10.1007/s13225-015-0330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodaparast S.A., Pourmoghaddam M.J., Amirmijani A., et al. Phylogenetic structure of the Iranian capnodiaceous sooty mould fungi inferred from the sequences of rDNA regions and TEF-1α. Mycological Progress. 2020;19:155–169. [Google Scholar]
- Kirk P.M., Cannon P.F., David J.C., et al. 9th edn. CAB International; Wallingford, UK: 2001. Ainsworth and Bisby’s Dictionary of the Fungi. [Google Scholar]
- Kirk P.M., Cannon P.F., Minter D.W., et al. 10th edn. CAB International; Wallingford, UK: 2008. Ainsworth and Bisby’s Dictionary of the Fungi. [Google Scholar]
- Léveillé J.H. Vol. 9. Victor Masson; Paris: 1847. Mycologie, mycétologie; pp. 261–303. (Dictionnaire Universel d’Historoire naturelle). [Google Scholar]
- Liu J.K., Hyde K.D., Jones E.G., et al. Fungal diversity notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Diversity. 2015;72:1–97. [Google Scholar]
- Liu Y.J., Whelen S., Hall B.D. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- Lücking R., Hodkinson B.P., Leavitt S.D. The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota - Approaching one thousand genera. The Bryologist. 2017;119:361–417. [Google Scholar]
- Lumbsch H.T., Lindemuth R. Major lineages of Dothideomycetes (Ascomycota) inferred from SSU and LSU rDNA sequences. Mycological Research. 2001;105:901–908. [Google Scholar]
- Luttrell E.S. The ascostromatic Ascomycetes. Mycologia. 1955;47:511–532. [Google Scholar]
- Maddison W.P., Maddison D.R. 2018. Mesquite: a modular system for evolutionary analysis.http://www.mesquiteproject.org Version 3.51. [Google Scholar]
- Miller M.A., Pfeiffer W., Schwartz T. Proceedings of the 1st conference of the extreme science and engineering discovery environment: bridging from the extreme to the campus and beyond. Association for Computing Machinery; USA: 2012. The CIPRES science gateway: enabling high-impact science for phylogenetics researchers with limited resources; pp. 1–8. [Google Scholar]
- Nylander J.A.A. Evolutionary Biology Centre, Uppsala University; Sweden: 2004. MrModeltest v2. Program distributed by the author. [Google Scholar]
- Quaedvlieg W., Binder M., Groenewald J.Z., et al. Introducing the consolidated species concept to resolve species in the Teratosphaeriaceae. Persoonia. 2014;33:1–40. doi: 10.3767/003158514X681981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaley A.W. Comminutispora gen. nov. and its Hyphospora gen. nov. anamorph. Mycologia. 1996;88:132–136. [Google Scholar]
- Rayner R.W. Commonwealth Mycological Institute and British Mycological Society; Kew, Surrey, UK: 1970. A mycological colour chart. [Google Scholar]
- Rehner S.A., Buckley E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia. 2005;97:84–98. doi: 10.3852/mycologia.97.1.84. [DOI] [PubMed] [Google Scholar]
- Ren S., Ma J., Zhang X. Two new Heteroconium species and two other forest microfungi newly recorded from China. Mycotaxon. 2012;119:361–367. [Google Scholar]
- Reynolds D.R. Observation on growth forms of sooty mold fungi. Nova Hedwigia. 1975;26:179–193. [Google Scholar]
- Reynolds D.R. Foliicolous ascomycetes: 3. The stalked capnodiaceous species. Mycotaxon. 1979;8:417–445. [Google Scholar]
- Reynolds D.R. Foliicolous ascomycetes 7. Phylogenetic systematics of the Capnodiaceae. Mycotaxon. 1986;27:377–403. [Google Scholar]
- Reynolds D.R. Capnodiaceous sooty mold phylogeny. Canadian Journal of Botany. 1998;76:2125–2130. [Google Scholar]
- Reynolds D.R., Gilbert G.S. Epifoliar fungi from Queensland, Australia. Australian Systematic Botany. 2005;18:265–289. [Google Scholar]
- Reynolds D.R., Gilbert G.S. Epifoliar fungi from Panama. Cryptogamie Myocologie. 2006;27:249–270. [Google Scholar]
- Rodríguez F.J., Oliver J.L., Marín A., et al. The general stochastic model of nucleotide substitution. Journal of Theoretical Biology. 1990;142:485–501. doi: 10.1016/s0022-5193(05)80104-3. [DOI] [PubMed] [Google Scholar]
- Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Ruíz R.F.C., Iturriaga T., Abarca G.H., et al. Notes on Heteroconium and a new species from Venezuela. Mycotaxon. 2008;105:175–184. [Google Scholar]
- Ruibal C., Gueidan C., Selbmann L., et al. Phylogeny of rock-inhabiting fungi related to Dothideomycetes. Studies in Mycology. 2009;64:123–133. doi: 10.3114/sim.2009.64.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch C.L., Crous P.W., Groenewald J.Z., et al. A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology. 2009;64:1–15. doi: 10.3114/sim.2009.64.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch C.L., Grube M. In: McLaughlin D.J., Spatafora J.W., editors. VII. Springer Verlag; Germany: 2015. Pezizomycotina: Dothideomycetes and arthoniomycetes; pp. 143–176. (The Mycota – systematics and evolution part B). [Google Scholar]
- Schoch C.L., Shoemaker R.A., Seifert K.A., et al. A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia. 2006;98:1041–1052. doi: 10.3852/mycologia.98.6.1041. [DOI] [PubMed] [Google Scholar]
- Sigler L., Tsuneda A., Carmichael J.W. Phaeotheca and Phaeosclera, two new genera of dematiaceous hyphomycetes and a redescription of Sarcinomyces Lindner. Mycotaxon. 1981;12:449–467. [Google Scholar]
- Smith C.W., Aptroot A., Coppins B.J., et al. British Lichen Society; London: 2009. The Lichens of Great Britain and Ireland. [Google Scholar]
- Stamatakis A. RAxML Version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1844–1849. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetrong S., Schoch C.L., Spatafora J.W., et al. Molecular systematics of the marine Dothideomycetes. Studies in Mycology. 2009;64:155–173. doi: 10.3114/sim.2009.64.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung G.-H., Sung J.-M., Hywel-Jones N.L., et al. A multigene phylogeny of Clavicipitaceae (Ascomycota, Fungi): identification of localized incongruence using a combinational bootstraap approach. Molecular Phylogenetics and Evolution. 2007;44:1204–1223. doi: 10.1016/j.ympev.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Van Nieuwenhuijzen E.J., Miadlikowska J.M., Houbraken J.A., et al. Wood staining fungi revealed taxonomic novelties in Pezizomycotina: new order Superstratomycetales and new species Cyanodermella oleoligni. Studies in Mycology. 2016;85:107–124. doi: 10.1016/j.simyco.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira S.I., Groenewald J.Z., Nakashima C., et al. Mycosphaerellaceae – chaos or clarity? Studies in Mycology. 2017;87:257–421. doi: 10.1016/j.simyco.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgalys R., Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T.J., Bruns T., Taylor J. In: A guide to molecular methods and applications. Innis M.A., Gelfand D.H., Sninsky J.J., White J.W., editors. Academic Press; New York: 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics; pp. 315–322. [Google Scholar]
- Wijayawardene N.N., Hyde K.D., Tibpromma S., et al. Towards incorporating asexual fungi in a natural classification: checklist and notes 2012–2016. Mycosphere. 2017;8:1457–1555. [Google Scholar]
- Woronichin N.N. Zur Kenntnis der Morphologie und Systematik der Russtaupilze Transkaukasiens. Annales Mycologici. 1926;24:231–264. [Google Scholar]
- Woronichin N.N. Über die Capnodiales. Annales Mycologici. 1925;23:174–178. [Google Scholar]
- Yamamoto W. Taxonomic studies on the Capnodiaceae 2. On the species of the Eucapnodiae. Annals of Phytopathological Society of Japan. 1954;19:1–5. [Google Scholar]
- Xu C., Zhang R., Sun G., et al. Comparative genome analysis reveals adaptation to the ectophytic lifestyle of sooty blotch and flyspeck fungi. Genome Biology and Evolution. 2017;9:3137–3151. doi: 10.1093/gbe/evx229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Ariyawansa H.A., Wu H.X., et al. The genus Leptoxyphium (Capnodiaceae) from China. Phytotaxa. 2014;176:174–183. [Google Scholar]
- Zalar P., de Hoog G.S., Gunde-Cimerman N. Taxonomy of the endoconidial black yeast genera Phaeotheca and Hyphospora. Studies in Mycology. 1999;43:49–56. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Original phylogenetic tree inferred from a Bayesian analysis based on a concatenated alignment of LSU, TEF-1α and RPB2. Bayesian posterior probabilities (BPP) and maximum likelihood bootstrap support values (MLBS) are indicated at the nodes (BPP/MLBS). The scale bar represents the expected number of changes per site. Families and orders are highlighted in blocks of different colour and indicated to the right of the tree. The tree was rooted with Venturia inaequalis (CBS 594.70).