Abstract
Objective
To describe 123I-FP-CIT (DAT scan) SPECT findings in progressive apraxia of speech (PAOS) patients and to compare those findings to progressive supranuclear palsy (PSP) and corticobasal syndrome (CBS).
Background
PAOS is a neurodegenerative syndrome in which patients present with apraxia of speech, a motor speech disorder affecting programming and planning of speech. Patients with PAOS predictably develop Parkinsonism. DAT scan is a neuroimaging tool that assesses the integrity of presynaptic dopamine transporters in basal ganglia and is usually abnormal in PSP and CBS.
Methods
As a part of an NIH-funded grant, we performed a DAT scan on 17 PAOS patients early in the disease course. DaTQUANT software was used to quantify uptake in left and right caudate and anterior/posterior putamen, with striatum to background ratios (SBRs). The PAOS cohort was compared to 15 PSP and 8 CBS patients.
Results
Five PAOS patients (29%) showed abnormalities in at least one striatal region on DAT scan. When the five PAOS patients with abnormal DAT was compared to the PSP and CBS patients, the only difference observed was lower uptake in the posterior putamen in PSP (p=0.03). There were no differences is putamen/caudate ratio or in symmetry of uptake, across all groups. There was also no difference in MDS-UPDRS-III scores between PAOS patients with and without abnormal DAT scans (p=0.56).
Conclusions
Abnormal DAT scan is observed early in the disease course in approximately 30% of PAOS patients, with striatal abnormalities similar to those in PSP and CBS.
Keywords: Progressive apraxia of speech (PAOS), progressive supranuclear palsy (PSP), corticobasal syndrome (CBS), Parkinsonism, Dopamine Scan, 123I-FP-CIT (DAT scan) SPECT
INTRODUCTION
Apraxia of speech (AOS) is a motor speech disorder of which the main problem is impaired planning and/or programming of speech [1]. Apraxia of speech may arise acutely after a left hemisphere stroke or may result from neurodegeneration, starting insidiously and progressing over time [2, 3]. Patients with progressive apraxia of speech (PAOS) sometimes can have other accompanying features that often are less severe, for example, agrammatic aphasia [4]. Whenever a patient presents solely with AOS in the absence of aphasia, the syndrome is referred to as primary progressive AOS (PPAOS) [5]. We have shown that in patients with PAOS, including those with PPAOS, motor parkinsonian symptoms, including bradykinesia and rigidity often develop over time [2, 6–9]. In one of our longitudinal studies of 13 PPAOS patients followed for an average of seven years from the disease onset, 10 developed parkinsonian symptoms, with a subset developing features that overlap with progressive supranuclear palsy (PSP) and corticobasal syndrome (CBS) within five years [2]. In keeping with this observation is the fact that PAOS patients commonly show tau pathology, either PSP or corticobasal degeneration at death [6].
Ioflupane 123I Dopamine transporter (DAT) SPECT was first approved by the FDA, in 2011, to differentiate Parkinson Disease from essential tremor given that DAT scans enable the visualization of presynaptic dopamine transporter function in the striatum and hence inform on the integrity of the nigrostriatal dopaminergic system [10, 11]. Patients with Parkinsonism including CBS and PSP show abnormalities on DAT scan with both CBS and PSP patients having loss of presynaptic dopamine transporter function [12–14].
Given the fact that PAOS patients commonly develop Parkinsonism with clinical features that overlap with those of PSPS and CBS, suggesting an involvement of the nigrostriatal dopaminergic system, we aimed to describe Ioflupane 123I DAT scan findings in PAOS, and also to compare DAT scan findings in PAOS to PSP and CBS. We hypothesized that DAT scan findings would be abnormal in at least some PAOS patients and show similar DAT scan abnormalities as in PSP and in CBS.
METHODS
Patient recruitment and evaluation
Patients with PAOS (n=17) were recruited by the Mayo Clinic, Neurodegenerative Research Group (NRG), between February 2018 and August 2019. All patients entered into an NIH-funded study and underwent a detailed speech and language, neurological and neuropsychological evaluation, and a DAT scan. DAT scan imaging was done in their first research visit. To be included in the study, all patients had to present with progressive AOS. Patients with concurrent illnesses that could account for the speech deficits, such as traumatic brain injury, stroke or developmental syndromes, and patients meeting criteria for another neurodegenerative disease, such as Alzheimer’s type dementia [15], dementia with Lewy bodies [16] behavioral variant frontotemporal dementia [17], probable PSP [18], CBS [19], multiple system atrophy [20], or motor neuron disease [21] were excluded.
The speech and language evaluation included the Apraxia of Speech Rating Scale (ASRS-3 score) [22] which determine the presence and prominence of a number of clinical features associated with AOS and the Western Aphasia Battery Aphasia Quotient (WAB-AQ) [23] to assess aphasia presence and severity. Judgments concerning the presence/absence of both AOS and aphasia were made by consensus between at least two speech-language pathologists based on reviewing video recordings of the entire speech and language examination, as previously described in detail [5]. The neurological and neuropsychological testing included the PSP Rating Scale [24] to assess the severity of PSP-related clinical features, the PSP Saccadic Impairment Scale (PSIS) [25] to assess eye movement abnormalities, the Movement Disorders Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale Parts (UPDRS-I, UPDRS-II, and UPDRS-III) [26] to assess motor function, the Limb Apraxia subscale of the WAB [23] to assess ideomotor apraxia, the Montreal Cognitive Assessment battery (MoCA) [23] to assess general cognition, the Frontal Behavioral Inventory (FBI) [27] to assess behavioral dyscontrol, and Trail Making Test A (TRAILS-A) [28] to assess visuomotor speed. A patient was judged to have Parkinsonism in the presence of bradykinesia plus an additional extrapyramidal finding: rigidity, postural instability, and resting tremor.
All PSP (n=15) and CBS (n=8) patients included in our study were seen clinically or for research purposes in the Department of Neurology, Mayo Clinic, Rochester, MN, by a Movement Disorder specialists (KAJ, HB, and FA) and had undergone DAT scan between January 2012 and October 2019. A DAT scan was performed in these patients to assess for confirmatory evidence of striatal abnormality.
Imaging
123I-FP-CIT scans (DAT scan, GE Healthcare, Chicago, IL) were acquired using a 5 mCi (±10%) dose in a GE Tandem Optima SPECT scanner equipped with a fan-beam collimator (GE Healthcare, Chicago, IL). After images were acquired, they were then reconstructed through the ordered subset expectation maximization (OSEM) method. No attenuation correction was used. In a recent clinico-pathological study in which patients had been scanned using fan-beam collimators prior to death, we found an area under the receiving operator curve (AUROC) value of 0.97 for DaTQUANT to discriminate between patients with and without a neurodegenerative disease involving the striatum [29]. Age-corrected Z-scores, quantifying the uptake of 123I-FP-CIT and striatum to background ratios (SBRs) for all striatal regions were individually calculated by DaTQUANT software, version 4.4, using a GE Healthcare Advantage Workstation. Results were obtained separately for the left and right anterior and posterior putamen and left and right caudate nucleus. Striatum to background rations were utilized to calculate a putamen/caudate mean uptake ratio for right and left anterior and posterior regions and absolute differences between right and left striatal regions to reflect the degree of asymmetry across hemispheres. The image selection and Z-score calculation for all patients were implemented by the same version of DaTQUANT. Although there is no definitely established threshold for abnormality, we selected a Z-score cut-off value of −1.5 to determine whether uptake was abnormal in at least one striatal region for the PAOS patients. In addition, for all 17 PAOS patients a nuclear medicine specialist had visually rated the DAT scan as being abnormal or normal, independent of the Z-score cut-point determination of abnormal.
Approvals
The study was approved by the Mayo Clinic Institutional Review Board (IRB approval number: 17–002468) and all patients consented for enrolment into the study.
Statistical analyses
Analyses were performed in JMP version 14 (SAS Institute, Cary, North Carolina, United States). Categorical data were summarized as counts and percentages, and continuous data were summarized as median and interquartile ranges (IQR). Statistical comparisons between two groups were performed using non-parametric Fisher’s Exact and Wilcoxon Rank sum tests conservatively assuming non-normalcy of the data. For comparison across more than two groups, we used the non-parametric Steel-Dwass-Critchlow-Fligner test to correct for multiple comparisons. The PAOS group was divided into those with normal DAT scan uptake in all striatal regions (PAOS-) and those with abnormal DAT scan uptake in at least one striatal region (PAOS+). Demographic and clinical variables, and DAT scan results, were compared between PAOS- and PAOS+ groups and as well as between PAOS-, PAOS+ and PSP and CBS. Alpha was set at P< 0.05.
RESULTS
Of the 17 PAOS patients, eight (47%) were male with the median age at onset of 70 years [Inter Quartile Range (IQR): 63–73] and disease duration (onset to scan) of 3 years (IQR: 1.5–5) (Table 1). Of the 15 PSP patients, 6 (40%) were male with median age at onset of 64 years (IQR: 56–66) and disease duration of median 2 years (IQR: 1–3). Of the eight CBS patients, 2 (25%) were male with median age at onset of 65 (IQR: 55–78) and disease duration of 3 years (IQR: 1.5–8) (Table 2).
Table 1:
Baseline demographic and clincial characteristics of the PAOS patients
| Variables | All PAOS (n=17) | PAOS− (n=12) | PAOS+ (n=5) | p-value |
|---|---|---|---|---|
| Sex [M] | 8 (47%) | 3 (25%) | 5 (100%) | 0.005* |
| Handedness [R] | 16 (94%) | 12 (100%) | 4 (80%) | 0.11 |
| Education, yrs. | 16 (13–18) | 16 (12.5–17.5) | 16 (12.5–19) | 0.55 |
| Age at onset, yrs. | 70 (63–73) | 70 (62.5–73) | 65 (55–78) | 0.83 |
| Age at DAT scan, yrs. | 73 (64–75) | 73 (63–75) | 69 (61–81) | 0.75 |
| Disease duration, yrs. | 3 (1.5–5) | 3 (1–5) | 4 (1.5–7) | 0.53 |
| UPDRS-I/52 | 7 (3.5–8) | 6 (4–8) | 7 (1.5–10.5) | 0.96 |
| UPDRS-II/52 | 3 (2.5–7) | 3 (2.25–7.25) | 4 (2.5–8) | 0.67 |
| UPDRS-III/132 | 11 (9–21.5) | 11.5 (10–22.75) | 11 (6.5–19.5) | 0.56 |
| FBI/72 | 10 (7.5–19.5) | 9 (6.25–11.75) | 18 (10.5–26) | 0.10 |
| Limb apraxia score/60 | 58 (52–59) | 58 (51.5–58) | 59 (53.5–59) | 0.33 |
| TRAILS-A MOANS | 8 (6–11.75) | 8 (6–11.75) | 7.5 (4.5–12) | 0.85 |
| ASRS-3 total | 19 (11–23.75) | 14 (10–24) | 23 (14–23.5) | 0.5 |
| Aphasia | 12 (71%) | 8 (67%) | 4 (80%) | 0.58 |
| AOS type (phonetic/prosodic)# | 7(41%)/4(24%) | 5(42%)/3(25%) | 2 (40%)/1(20%) | 0.96 |
| MoCA/30 | 25 (19–26) | 24 (19–27) | 25 (18–28) | 0.91 |
| WAB-AQ/100 | 96 (92.5–97.6) | 95 (92.5–97.5) | 97 (81–99) | 0.4 |
| PSIS/5 | 1 (0–1) | 0.5 (0–1) | 1 (0.5–1.5) | 0.3 |
| PSP-RS/100 | 12 (7–17) | 11 (7–18.5) | 12 (6–19.5) | 0.9 |
| PSP-GM/20 | 1 (0–3) | 1 (0–2.75) | 0 (0–3.5) | 0.8 |
Statistically significant.
Remaining cases had mixed features of both prosodic and phonetic
Data for sex, handedness, aphasia, and AOS type summarized as numbers (percentages) and for all other variables summarized as median (interquartile ranges). [M] represents male sex.
PAOS− means PAOS patients with normal DAT scan, PAOS+ means PAOS patients with abnormal DAT scan.
Table 2:
Comparison of demographic and DAT measures in PAOS, CBS, and PSP patients
| Variables | PAOS− (n=12) | PAOS+ (n=5) | CBS (n=8) | PSP (n=15) | PAOS+ vs. PAOS− p-value | PAOS+ vs. CBS p-value | PAOS+ vs. PSP p-value |
|---|---|---|---|---|---|---|---|
| Sex [M] | 3 (25) | 5 (100) | 2 (25) | 6 (40) | 0.04* | 0.07 | 0.12 |
| Age at onset | 70 (62.5, 73) | 65 (55, 78) | 54 (49,62) | 64 (56, 66) | 1 | 0.5 | 0.82 |
| Age at DAT scan | 73 (63, 75) | 69 (61, 81) | 60 (57, 64) | 66 (62, 71) | 0.99 | 0.41 | 0.72 |
| Disease duration | 3 (1, 5) | 4 (1.5, 7) | 3 (1.5, 8) | 2 (1, 3) | 0.94 | 1 | 0.86 |
| Right anterior Putamen | 1.89 (1.73, 2.42) | 1.33 (0.88, 1.73) | 1.57 (0.90, 1.82) | 0.82 (0.60, 1.08) | 0.08 | 0.99 | 0.22 |
| Left anterior Putamen | 1.8 (1.62, 2.40) | 1.30 (0.97, 1.44) | 1.55 (1.05, 2.1) | 0.8 (0.50, 0.94) | 0.01* | 0.7 | 0.07 |
| Right posterior putamen | 1.70 (1.38, 2.28) | 1.21 (0.66, 1.50) | 1.36 (0.53, 1.70) | 0.59 (0.35, 0.70) | 0.17 | 0.99 | 0.10 |
| Left posterior Putamen | 1.68 (1.42, 2.33) | 1.04 (0.90, 1.18) | 1.25 (0.63, 1.91) | 0.42 (0.30, 0.56) | 0.05* | 0.98 | 0.03* |
| Right caudate | 2.63 (2.2, 2.70) | 1.48 (1.40, 2.30) | 1.94 (1.11, 2.13) | 1.24 (0.75, 1.55) | 0.17 | 0.99 | 0.18 |
| Left caudate | 2.25 (2.10, 2.64) | 1.73 (1.42, 2.17) | 1.86 (1.54, 2.54) | 1.18 (0.70, 1.29) | 0.13 | 0.91 | 0.08 |
| Right anterior putamen/caudate ratio | 0.84 (0.75, 0.88) | 0.73 (0.62, 0.84) | 0.82 (0.78, 0.87) | 0.71 (0.60, 0.83) | 0.55 | 0.69 | 0.99 |
| Left anterior putamen/ caudate ratio | 0.80 (0.76, 0.90) | 0.75 (0.57, 0.83) | 0.79 (0.68, 0.84) | 0.71 (0.61, 0.78) | 0.42 | 0.85 | 0.99 |
| Right posterior putamen/caudate ratio | 0.72 (0.63, 0.85) | 0.57 (0.47, 0.81) | 0.70 (0.46, 0.82) | 0.48 (0.31, 0.67) | 0.55 | 0.96 | 0.90 |
| Left posterior putamen/ caudate ratio | 0.79 (0.66, 0.85) | 0.58 (0.46, 0.80) | 0.65 (0.41, 0.76) | 0.43 (0.31, 0.70) | 0.68 | 1 | 0.61 |
| Anterior putamen absolute difference | 0.12 (0.08, 0.26) | 0.27 (0.24, 0.37) | 0.12 (0.03, 0.23) | 0.12 (0.08, 0.19) | 0.23 | 0.14 | 0.1 |
| Posterior putamen absolute difference | 0.13 (0.04, 0.18) | 0.34 (0.22, 0.41) | 0.14 (0.03, 0.32) | 0.18 (0.07, 0.35) | 0.06 | 0.41 | 0.26 |
| Caudate absolute difference | 0.28 (0.12, 0.32) | 0.24 (0.13, 0.37) | 0.29 (0.05, 0.53) | 0.16 (0.11, 0.23) | 0.96 | 0.88 | 0.22 |
Statistically significant
Data for sex summarized as numbers (percentages) and for all other variables summarized as median (interquartile ranges). [M] represents the male sex.
PAOS− means PAOS patients with normal DAT scan, PAOS+ means PAOS patients with abnormal DAT scan
In the 17 PAOS patients, five showed reduced striatal radioisotope binding on the DAT scan. Three (60%) showed lower uptake in both anterior and posterior putamen equally, one showed lower uptake only in the left posterior putamen and one only in the left anterior putamen; Caudate uptake was also abnormal in one of the patients who showed both anterior and posterior putamen involvement.
When all 17 PAOS patients were compared to the 15 PSP patients, the PSP group showed lower radioisotope uptake throughout the striatum on both sides (p<.001 for all). However, when the PAOS patients were compared to the 8 CBS patients, there were no significant differences in any region.
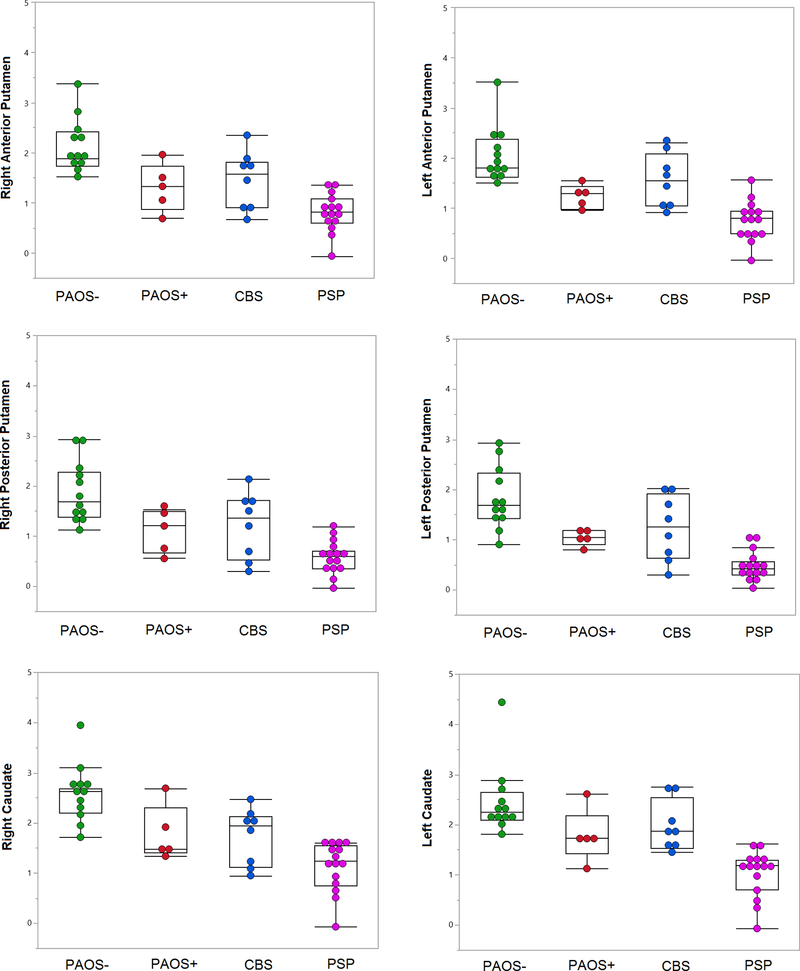
When SBR values in 12 PAOS- patients were compared to the 5 PAOS+ patients, the latter group showed significantly lower uptake in the left anterior and posterior putamen (p=0.01 and p=0.05) (Table 2). For the remaining striatum, there was no significant difference between the two groups. Although not statistically significant, PAOS- tended to have higher uptake throughout the striatum than PAOS+ (Table 2) (Figure 1). In fact, PAOS- patients had significantly higher SBRs than PSP patients in all basal ganglia regions (p<.001 for all). However, there was no significant difference than CBS patients in all basal ganglia regions, but right caudate (p=0.03). When putamen/caudate ratios of PAOS+ patients compared to PAOS-, there were no significant differences across the groups for right and left anterior and posterior putamen. Right-left absolute differences in anterior and posterior putamen and caudate of PAOS+ group were not different from PAOS- (Table 2).
Figure 1:
Box plots are demonstrating the greatest and the lowest striatum to background ratios (SBRs), lower quartile (25th percentile), upper quartile (75th percentile), and median SBR values for each group. Each dot represents each patient.
When SBR values in the 5 PAOS+ patients were compared to PSP and CBS, only left posterior putamen (p=0.03) had lower uptake in the PSP group compared to the PAOS+ group. No significant differences were observed between PAOS+ and CBS (Table 2). Even though not statistically significant, PSP patients in general tended to have the lowest uptake in all striatal regions compared to PAOS+ and to CBS (Table 2) (Figure 1). There were also no significant differences for putamen/caudate ratios or for absolute asymmetry between PAOS+ and both PSP and CBS (Table 2) Representative DAT scans for all four groups of patients are shown in Figure 2.
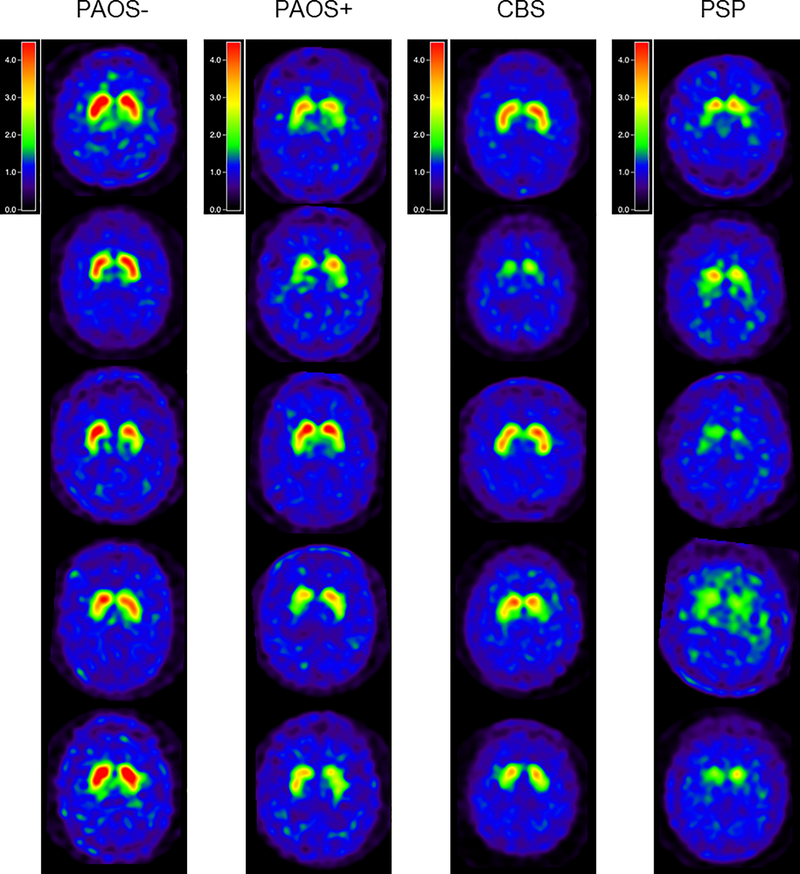
Figure 2:
DAT scan images from five representative PAOS+, CBS and PSP patients normalized to the occipital value. Note the severe loss of striatal uptake in some PSP patients compared to loss in the PAOS+ and CBS patients.
The only difference in baseline clinical characteristic features was the sex. The PAOS+ group had a higher proportion of men compared to the PAOS- group (p=0.005) (Table 1). No differences were observed between the PAOS+ and PAOS- groups for any of the other clinical measures, including total MDS-UPDRS-III scores (p=0.56) (Table 1). Absent-mild, mild, and mild-moderate bradykinesia were observed in 7 of 17 PAOS patients (41%) at baseline visit. Of these seven patients, two were PAOS+ (40%) and five were PAOS- (41%). Rigidity was observed in two of the 17 PAOS patients (12%), one being PAOS+ the other PAOS-. Postural instability was observed only in one patient. None of our PAOS patients had resting tremor. In total, 3 patients demonstrated mild clinical Parkinsonism of which one was PAOS+ (20%) (Table 3).
Table 3-.
Parkinsonian signs on examination in all 17 PAOS patients
| Patients╲Clinical features | DAT scan status | Bradykinesia | Rigidity | Postural instability | Resting tremor | Parkinsonism |
|---|---|---|---|---|---|---|
| 1¥ | − | Yes | No | No | No | No |
| 2 | − | No | No | No | No | No |
| 3 | − | No | No | No | No | No |
| 4 | − | No | No | No | No | No |
| 5 | − | No | No | No | No | No |
| 6β | − | Yes | No | Yes | No | Mild |
| 7 | − | No | No | No | No | No |
| 8ф | − | Yes | No | No | No | No |
| 9 | − | No | No | No | No | No |
| 10Ω | − | Yes | Yes | No | No | Mild |
| 11 | − | No | No | No | No | No |
| 12β | − | Yes | No | No | No | No |
| 13 | + | No | No | No | No | No |
| 14 | + | No | No | No | No | No |
| 15β | + | Yes | No | No | No | No |
| 16 | + | No | No | No | No | No |
| 17Ω | + | Yes | Yes | No | No | Mild |
Bradykinesia restricted to lower extremities of mild-moderate severity
Bradykinesia mild-moderate in upper extremities and mild in lower extremities
Bradykinesia mild-moderate in upper extremities, absent-mild in lower extremities and mild rigidity in upper extremities
Bradykinesia mild in upper extremities only
Parkinsonism is determined by the presence of bradykinesia plus one of the other clinical features (rigidity, postural instability, and resting tremor).
DISCUSSION
In this study, we found that almost a third of PAOS patients have an abnormal DAT scan and hence abnormal presynaptic dopamine transporter function. We found that there was little difference in striatal uptake abnormality between PAOS patients with DAT abnormalities and patients with PSP and CBS. There appeared to be a dissociation between MDS-UPDRS-III scores and DAT scan abnormalities, in PAOS.
Five of the 17 PAOS patients had abnormalities in at least one striatal region on the DAT scan, demonstrating that DAT abnormalities are indeed observed in this population. DAT scan abnormalities of the five PAOS+ patients were not very different from the PSP and CBS patients. This is not surprising given that most PAOS patients progress over time and develop Parkinsonism with features that overlap with those of PSP and CBS [2, 8, 9]. We did note, however, a trend for the PSP patients to have the lowest striatal uptake of all the groups, with significantly lower uptake observed in the left posterior putamen. There was no significant difference in SBRs between PAOS+ and CBS in any of the striatal regions and there was no trend for CBS to show less receptor binding. Hence, the PAOS+ patients appeared slightly more similar to CBS than PSP. Our PAOS patients with abnormal DAT scans showed similar involvement of both anterior and posterior putamen, as has also been observed in PSP [30] and CBS [31]. Furthermore, the PAOS+ patients did not demonstrate significantly more asymmetry than CBS and PSP. CBS has been found to be more asymmetric when compared to normal controls but not when compared to PSP [12]. Hence, it not surprising that PAOS was not any more or less asymmetric when compared to PSP and CBS. We did not find the putamen/caudate ratio to be helpful to differentiate the PAOS+ group from any other group, including the PAOS- group.
Given the mechanism of DAT abnormalities in Parkinsonism, it appears that early striatal dysfunction is a feature of some PAOS patients. However, only one of the PAOS+ patients was clinically considered to have Parkinsonism and the PAOS+ patients with abnormal DAT scans did not demonstrate more Parkinsonism on the MDS-UPDRS-III scale compared to the PAOS- patients. Hence, it would appear that DAT scan is a more sensitive detector of early striatal abnormalities in PAOS compared to clinical examination. DAT scan has also been found to be an early marker of Parkinsonism in patients with rapid eye movement sleep behavior disorder who later developed an alpha-synucleinopathy [32].
Somewhat unexpectedly, the PAOS+ patients with abnormal DAT scans did not demonstrate more Parkinsonism on the MDS-UPDRS-III scale compared to the PAOS- patients. Similarly, the proportion of patients who demonstrated clinical Parkinsonism was almost equal for both the PAOS- and PAOS+ groups. This brings up the issue of patients diagnosed with idiopathic Parkinson’s disease without evidence of dopaminergic deficit (SWEDD). Such patients were later found to have alternative clinical diagnoses [33–35]. One of the reasons for the low association in our PAOS cohort was that three of the PAOS- patients had high MDS-UPDRS-III scores [range: 24–36]. This result indicates that the high MDS-UPDRS-III scores in these three patients do not reflect involvement of the presynaptic dopamine transporters. Hence, other causes of elevated scores needed to be explored. On review of the imaging studies in these three patients, there was no evidence of normal pressure hydrocephalus or vascular pathology. There was also no evidence for drug-induced Parkinsonism, psychogenic Parkinsonism, or other metabolic causes to explain the high MDS-UPDRS-III scores and the normal DAT scans. There was also no difference in limb praxis measures that could have affected performance on the MDS-UPDRS-III. In looking at the individual MDS-UPDRS-III scores in these three PAOS- patients it appears that the higher scores were driven mainly by bradykinetic alternating motion rates of the limbs without motor arrest. We hypothesize that bradykinetic phenomenon in PAOS could reflect cortical involvement. This hypothesis is predicated on the fact that some PAOS patients have AOS characteristics of predominantly slow and segmented speech known as AOS type 2[36] or prosodic AOS[37]. Such patients show involvement of the medial and lateral premotor cortex on neuroimaging measures including MRI and [18F] fluorodeoxyglucose PET scans [5, 37]. Future studies are needed to test this hypothesis.
There are a few limitations of this study including the cross-sectional study design. We are, however, currently following all 17 PAOS patients and in the future will be able to determine how the DAT scan and MDS-UPDRS-III scores and relationships between them evolve. Another limitation is the lack of pathological diagnosis of patients in the cohort. Third, our sample size was relatively small. Lastly, we did not have a normal control group with fan-beam collimators as a comparison group.
CONCLUSION
Abnormal DAT scan can be observed early in the disease course in approximately 30% of PAOS patients, with abnormalities similar to those observed in PSP and CBS. DAT scan, therefore, has potential as an early diagnostic measure of impaired dopamine transporter integrity that might be more sensitive than clinical measures including the MDS-UPDRS-III scale.
Acknowledgment
Financial disclosure: This project is funded by NIH grants R01 DC14942 and R01 DC12519.
Footnotes
Summary conflict of interest: No conflicts exist for any of the authors.
REFERENCES
- 1.McNeil MR, Robin DA, and Schmidt RA, Apraxia of speech: Definition and differential diagnosis. Clinical management of sensorimotor speech disorzers, 2009. 2: p. 249–267. [Google Scholar]
- 2.Josephs KA, et al. , The evolution of primary progressive apraxia of speech. Brain, 2014. 137(Pt 10): p. 2783–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffy JR, Apraxia of speech in degenerative neurologic disease. Aphasiology, 2006. 20(6): p. 511–527. [Google Scholar]
- 4.Josephs KA, et al. , Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase, 2005. 11(4): p. 283–96. [DOI] [PubMed] [Google Scholar]
- 5.Josephs KA, et al. , Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain, 2012. 135(Pt 5): p. 1522–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Josephs KA, et al. , Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain, 2006. 129(Pt 6): p. 1385–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitwell JL, et al. , An Evaluation of the Progressive Supranuclear Palsy Speech/Language Variant. Mov Disord Clin Pract, 2019. 6(6): p. 452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tetzloff KA, et al. , Clinical and imaging progression over 10 years in a patient with primary progressive apraxia of speech and autopsy-confirmed corticobasal degeneration. Neurocase, 2018. 24(2): p. 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Utianski RL, et al. , Clinical Progression in Four Cases of Primary Progressive Apraxia of Speech. Am J Speech Lang Pathol, 2018. 27(4): p. 1303–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall VL, et al. , Parkinson’s disease is overdiagnosed clinically at baseline in diagnostically uncertain cases: a 3-year European multicenter study with repeat [123I]FP-CIT SPECT. Mov Disord, 2009. 24(4): p. 500–8. [DOI] [PubMed] [Google Scholar]
- 11.Seifert KD and Wiener JI, The impact of DaTscan on the diagnosis and management of movement disorders: A retrospective study. Am J Neurodegener Dis, 2013. 2(1): p. 29–34. [PMC free article] [PubMed] [Google Scholar]
- 12.Pirker W, et al. , [123I]beta-CIT SPECT in multiple system atrophy, progressive supranuclear palsy, and corticobasal degeneration. Mov Disord, 2000. 15(6): p. 1158–67. [DOI] [PubMed] [Google Scholar]
- 13.Klaffke S, et al. , Dopamine transporters, D2 receptors, and glucose metabolism in corticobasal degeneration. Mov Disord, 2006. 21(10): p. 1724–7. [DOI] [PubMed] [Google Scholar]
- 14.Im JH, et al. , Differential patterns of dopamine transporter loss in the basal ganglia of progressive supranuclear palsy and Parkinson’s disease: analysis with [(123)I]IPT single photon emission computed tomography. J Neurol Sci, 2006. 244(1–2): p. 103–9. [DOI] [PubMed] [Google Scholar]
- 15.McKhann G, et al. , Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology, 1984. 34(7): p. 939–44. [DOI] [PubMed] [Google Scholar]
- 16.McKeith IG, et al. , Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology, 2005. 65(12): p. 1863–72. [DOI] [PubMed] [Google Scholar]
- 17.Neary D, et al. , Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology, 1998. 51(6): p. 1546–54. [DOI] [PubMed] [Google Scholar]
- 18.Litvan I, et al. , Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology, 1996. 47(1): p. 1–9. [DOI] [PubMed] [Google Scholar]
- 19.Boeve BF, Lang AE, and Litvan I, Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol, 2003. 54 Suppl 5: p. S15–9. [DOI] [PubMed] [Google Scholar]
- 20.Gilman S, et al. , Second consensus statement on the diagnosis of multiple system atrophy. Neurology, 2008. 71(9): p. 670–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks BR, et al. , El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord, 2000. 1(5): p. 293–9. [DOI] [PubMed] [Google Scholar]
- 22.Duffy JR, Strand EA, and Josephs KA, Motor Speech Disorders Associated with Primary Progressive Aphasia. Aphasiology, 2014. 28(8–9): p. 1004–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasreddine ZS, et al. , The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc, 2005. 53(4): p. 695–9. [DOI] [PubMed] [Google Scholar]
- 24.Hoglinger GU, et al. , Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord, 2017. 32(6): p. 853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitwell JL, et al. , Clinical correlates of white matter tract degeneration in progressive supranuclear palsy. Arch Neurol, 2011. 68(6): p. 753–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goetz CG, et al. , Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord, 2008. 23(15): p. 2129–70. [DOI] [PubMed] [Google Scholar]
- 27.Kertesz A, Davidson W, and Fox H, Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci, 1997. 24(1): p. 29–36. [DOI] [PubMed] [Google Scholar]
- 28.Strauss SA, An unusual case of wrongful pregnancy: liability of doctor resulting from misrepresentation. Med Law, 1998. 17(1): p. 7–11. [PubMed] [Google Scholar]
- 29.Maltais DD, et al. , Confirmation of (123)I-FP-CIT-SPECT (ioflupane) quantification methods in dementia with Lewy body and other neurodegenerative disorders. J Nucl Med, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antonini A, et al. , 123I-Ioflupane/SPECT binding to striatal dopamine transporter (DAT) uptake in patients with Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Neurol Sci, 2003. 24(3): p. 149–50. [DOI] [PubMed] [Google Scholar]
- 31.Badoud S, et al. , Discriminating among degenerative parkinsonisms using advanced (123)I-ioflupane SPECT analyses. Neuroimage Clin, 2016. 12: p. 234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iranzo A, et al. , Dopamine transporter imaging deficit predicts early transition to synucleinopathy in idiopathic rapid eye movement sleep behavior disorder. Ann Neurol, 2017. 82(3): p. 419–428. [DOI] [PubMed] [Google Scholar]
- 33.Stephenson D, et al. , The Qualification of an Enrichment Biomarker for Clinical Trials Targeting Early Stages of Parkinson’s Disease. J Parkinsons Dis, 2019. 9(3): p. 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicastro N, Burkhard PR, and Garibotto V, Scan without evidence of dopaminergic deficit (SWEDD) in degenerative parkinsonism and dementia with Lewy bodies: A prospective study. J Neurol Sci, 2018. 385: p. 17–21. [DOI] [PubMed] [Google Scholar]
- 35.Marek K, et al. , Longitudinal follow-up of SWEDD subjects in the PRECEPT Study. Neurology, 2014. 82(20): p. 1791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Josephs KA, et al. , Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology, 2013. 81(4): p. 337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Utianski RL, et al. , Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain Lang, 2018. 184: p. 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]