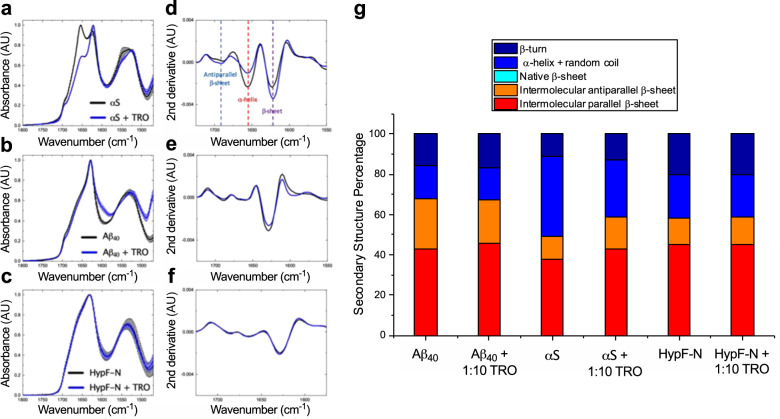
Fig. 5. Structural characterization of the various types of oligomers with a 10-fold excess of trodusquemine.
IR absorbance measurements of oligomers of αS (a), Aβ40 (b), and HypF-N (c) incubated in the absence (black) or presence of a 10-fold excess of trodusquemine (blue). The spectra were acquired in triplicate and averaged and the error bars indicate the s.e.m. of n = 3 replicates corresponding to independent protein depositions of the same sample. All spectra were normalized to assign an arbitrary value of 1.0 AU to the maximum absorbance. Corresponding secondary derivative analysis of the averaged spectra for αS (d), Aβ40 (e), and HypF-N (f), with key inflection points corresponding to antiparallel β-sheet (light blue), α-helix (red), and parallel β-sheet (purple) indicated with vertical dashed lines. g FTIR-derived secondary structure composition of the oligomers. Spectra were analyzed using a 2nd order, 12 point Savitzky-Golay filter. The presence of a 10-fold excess of trodusquemine was observed to increase the β-sheet content and reduce the combined α-helix and random coil content of αS oligomers, while the overall structural compositions of Aβ40 and HypF-N oligomers were largely unchanged by the presence of the molecule.