Figure 7.
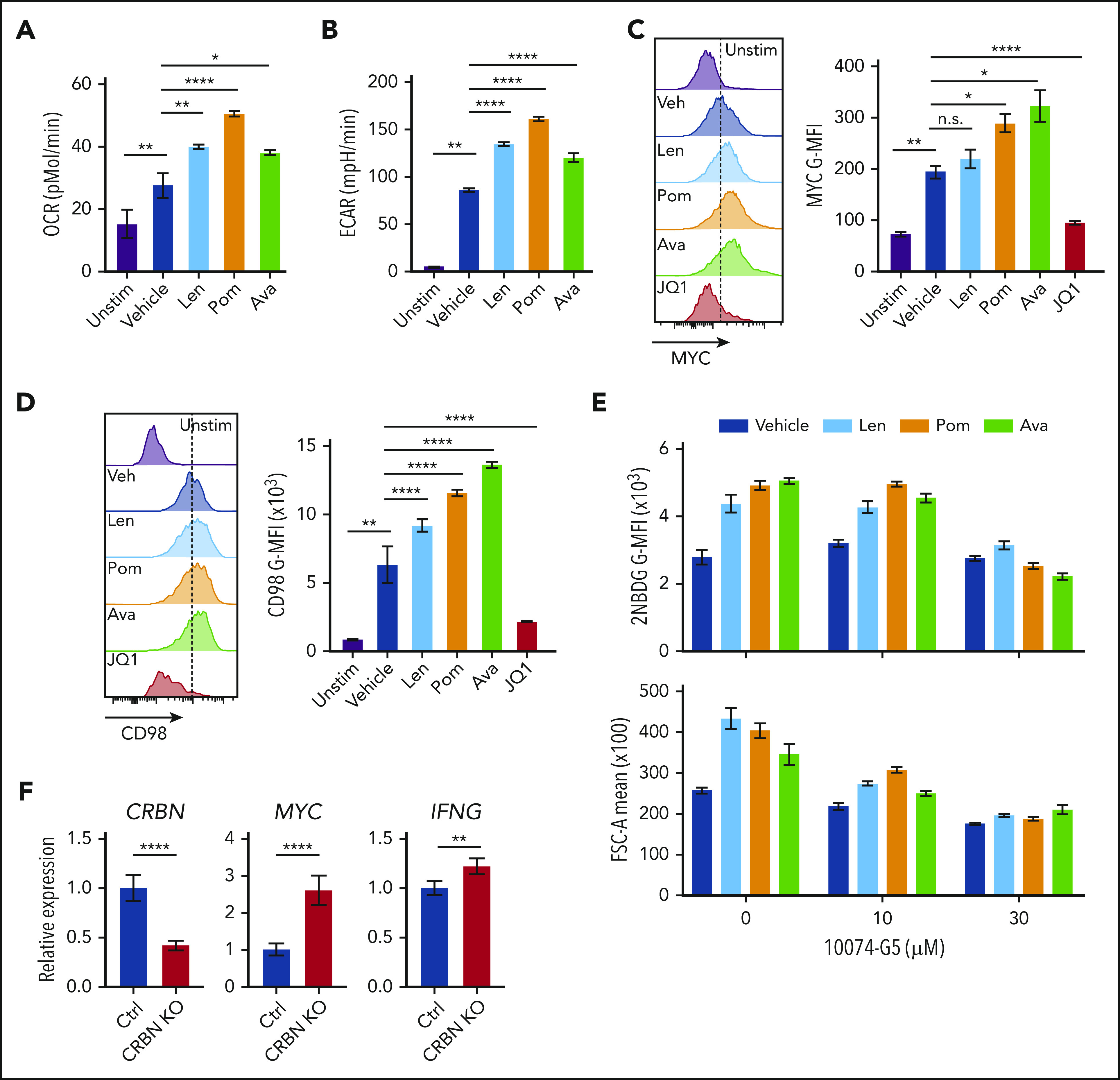
Targeting CRBN augments human CD8+ T-cell bioenergetics and MYC expression. OCRs (A) and ECARs (B) in activated human CD8+ T cells treated with vehicle or IM compounds for 5 days, as indicated, at a 10-µM dose of lenalidomide (Len), pomalidomide (Pom), and avadomide (Ava). (C) MYC protein levels in unstimulated (Unstim) and anti-CD3+anti-CD28–activated human CD8+ T cells treated with IM compounds (Len, Pom or Ava; 10 µM) for 5 days as detected by flow cytometry. JQ1 (a BET inhibitor) was used to show staining specificity for MYC in these experiments. (D) CD98 surface expression in activated human CD8+ T cells treated with IM compounds for 5 days. (E) Glucose uptake (top) and cell size (bottom) of activated (treated with anti-CD3+anti-CD28) human CD8+ T cells treated with IM compounds, with or without the MYC/MAX dimerization inhibitor 10074-G4, as measured using the fluorescent glucose analogue 2-NBDG (top) or forward scatter area (FSC-A, bottom) by flow cytometry. (F) Resting CD8+ T cells, enriched from healthy donor peripheral blood mononuclear cell (PBMCs), and complexed ribonucleoproteins (CRBN gRNAs+CAS9) were mixed with 4 × 106 CD8+ T cells, resuspended in primary cell solution, and nucleoporated. After nucleoporation, the cells were rested for 48 hours and then activated with anti-CD3+anti-CD28. After 5 days, the cells were assessed for levels of CRBN, MYC, and IFNg mRNA vs B2M transcripts by qRT-PCR. All results are representative of at least 2 independent experiments (excluding the CRISPR experiments). For statistical comparisons, see supplemental Table 5. n.s., not significant; *P < .05; **P < .01; ****P < .0001.
