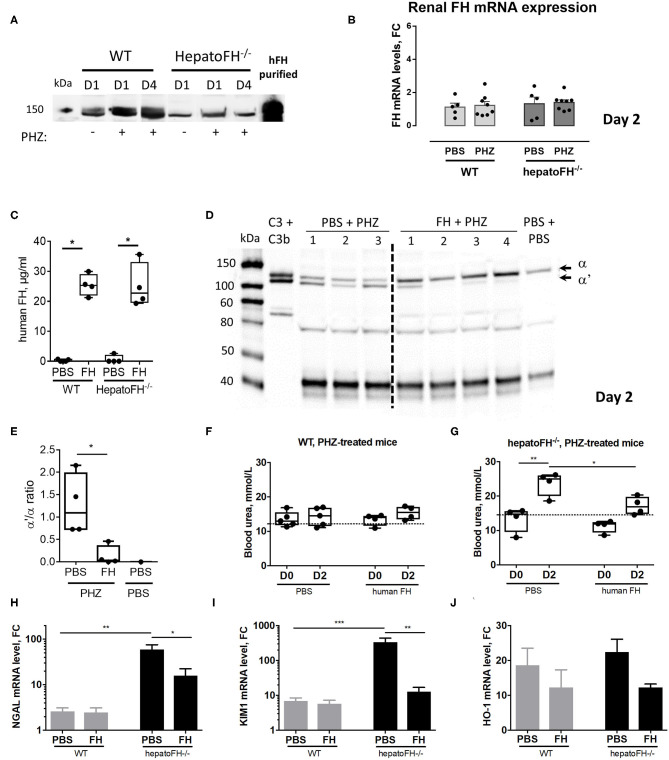
Figure 5.
Purified FH controls kidney injury in hemolytic conditions. (A) Measurement of plasma FH by WB after PBS or PHZ injection (Day 1 or 4 post-injection) in WT and hepatoFH−/− mice. Each lane correspond to 1 individual mouse. (B) Assessment of mRNA renal expression of FH in total kidney tissue. (C) Plasma human FH was assessed by ELISA at day 2 in WT and hepatoFH-/- mice, injected with human FH or PBS. (D) Plasma of hepatoFH−/− mice was resolved by electrophoresis and probed for C3 cleavage to C3b in hepatoFH−/− mice, treated or not with purified human FH. Each lane correspond to one individual mouse. (E) Quantification of the α'/α bands ratio, showing the absence of C3 activation in case of FH administration. (F,G) Blood urea was measured in plasma samples collected from PHZ-injected WT (F) or hepatoFH−/− (G) mice, pre-treated with human FH or PBS, at day 0 (D0) and day 2 (D2). (H–J) Assessment of mRNA renal expression for NGAL (Lcn2) (H), Kim-1 (Havcr1) (I) and HO-1 (Hmox1) (J) at day 2 in PHZ-treated WT and hepatoFH−/− mice pre-treated with human FH or PBS. P-values are derived from Two way ANOVA test with Sidak correction for multiple comparisons: *p < 0.05; **p < 0.005; ***p < 0.001. Plasma parameters are box plots and dots with median and Min/Max points. mRNA values are represented in fold change (FC), relatively to the mean expression of PBS-treated mice for each strain. Values are represented as mean +/- SEM for the gene expression and SD for the remaining panels. Data obtained from one experiment, experimental groups containing 4 mice.