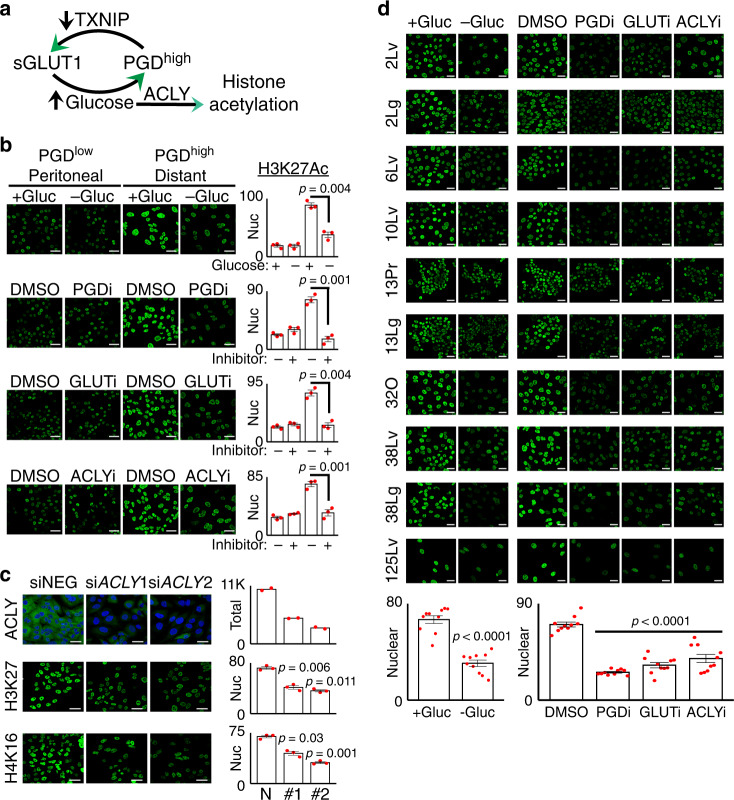
Fig. 5. A PGD-GLUT1-ACLY pathway hyperacetylates metastatic chromatin.
a Illustration of how PGD-driven retention of surface GLUT1 can provide excess glucose for ACLY-dependent histone hyperacetylation. b Glucose deprivation (−Gluc, top row), PGD inhibition (PGDi, second row), GLUT1 inhibition (GLUTi, third row), and ACLY inhibition (ACLYi, bottom row) all reduced global H3K27Ac in the nuclei of PGDhigh 38Lg cells (right panels) down to levels comparable with PGDlow 38Per controls from the same patient (left panels) by confocal IF. c Treatment of PGDhigh 38Lg cells with siRNAs against ACLY (#1, #2) knocked down ACLY expression (top panels, n = 2 biological replicates) and reduced histone acetylation (H3K27Ac: middle panels;, H4K16Ac: bottom panels; n = 3 biological replicates) relative to nontargeting siRNA controls (siNEG). d Glucose deprivation, PGDi, GLUT1i, and ACLYi recurrently lowered H3K27Ac across the full complement of PGDhigh subclones (left-hand labels). Quantified nuclear H3K27Ac values for all the indicated experiments pooled together are plotted at the bottom. For all IF experiments (unless otherwise indicated): green, antibody signal, n = 3 biological replicates, error bars: s.e.m., indicated p values calculated by two-tailed t tests, scale bars: 20 µm.