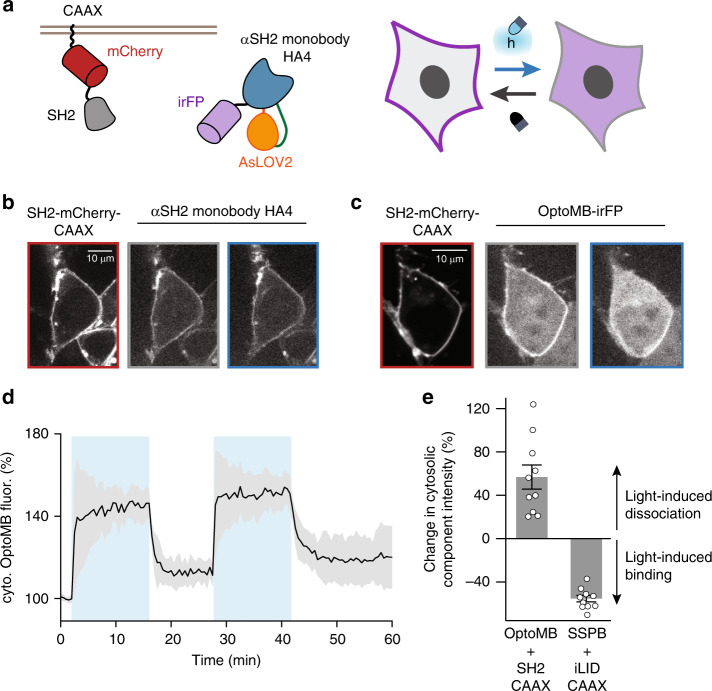
Fig. 5. Characterization of OptoMB in mammalian cells.
a Schematic diagram of the membrane-binding assay used, where irFP-tagged OptoMB (blue–orange–purple) binds to a fusion of SH2 (gray), mCherry (red), and CAAX (black), anchored to the plasma membrane (PM) of HEK293T cells. In the dark, OptoMB-irFP binds to the membrane-bound SH2, enhancing irFP signal at the PM and reducing it in the cytosol. In blue light, OptoMB releases SH2, causing a reduction in irFP signal at the membrane and an increase in the cytosol. b, c Images represent two replicate experiments. HEK293T cells expressing SH2-mCherry-CAAX and irFP-tagged HA4 monobody (b) or OptoMB-iRFP (c) imaged in the dark or blue light. Left panels (red) show mCherry fluorescence of SH2 fusion anchored to the PM; central (gray) and right (blue) panels show HA4-irFP (b) or OptoMB-iRFP (c) fluorescence in the dark and after 30 s of blue light stimulation, respectively, showing irFP-HA4 localized to the PM in either light condition (b) or OptoMB enriched at the PM in the dark and in the cytosol in the light (c). White scale bar in the cell images is 10 μm. d Cytosolic OptoMB-iRFP fluorescence changing over time due to periodic pulses of blue light (blue sections). The fluorescence is expressed in percentage from the original value of cells in the dark. Curve and shaded regions indicate mean ± SD for at least 10 cells respectively. e Comparison of light-induced translocation by OptoMB and iLID-SSPB. Error bars indicate mean ± SEM for n = 10 cells per variant. For iLID/SSPB translocation, cytosolic BFP fluorescence was measured using NIH3T3 cells with a stably integrated BFP-SSPB-SOScat-P2A-iLID-CAAX lentiviral construct. Source data are provided as a Source Data file.