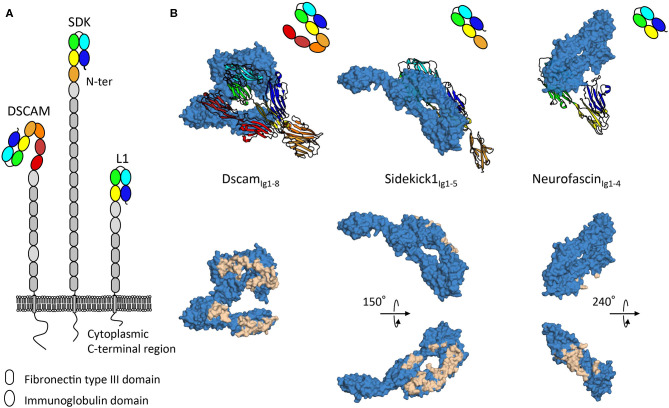
Figure 2.
Architecture and homophilic adhesion interfaces of horseshoe containing subfamilies of the immunoglobulin superfamily. (A) Type-I transmembrane extracellular DSCAM, sidekick, and L1 protein families' architecture with modular domain organization comprising Ig-like and fibronectin type III repeats. (B) Crystal structures of homophilic binding modules of DSCAM, sidekick1, and neurofascin from DSCAM, sidekick, and L1 protein families. Top panels show how horseshoes use different faces for interactions. The ribbon copy is held in the same orientation while the space filling copy interacts with distinct faces. Bottom panels show interacting residues plotted on the surface of the space filled copy of the molecules, displaying extensive interfaces on distinct domain faces.