Abstract
Background
Limited evidence exists regarding the cost and health-related quality of life (HRQoL) effects of non–muscle-invasive bladder cancer (NMIBC) recurrence and progression to muscle-invasive bladder cancer (MIBC). We examined these effects using evidence from a recent randomized control trial.
Material and Methods
The costs and HRQoL associated with bladder cancer were assessed using data from the BOXIT trial (bladder COX-2 inhibition trial; n = 472). The cost and HRQoL effects from clinical events were estimated using generalized estimating equations. The costs were derived from the recorded resource usage and UK unit costs. HRQoL was assessed using the EQ-5D-3L and reported UK preference tariffs. The events were categorized using the TMN classification.
Results
Cases of grade 3 recurrence and progression were associated with statistically significant HRQoL decrements (−0.08; 95% confidence interval [CI], −0.13 to −0.03; and −0.10; 95% CI, −0.17 to −0.03, respectively). The 3-year average cost per NMIBC patient was estimated at £8735 (95% CI, 8325-9145). Cases of grade 1, 2, and 3 recurrence were associated with annual cost effects of £1218 (95% CI, 403-2033), £1677 (95% CI, 920-2433), and £3957 (95% CI, 2332-5583), respectively. Progression to MIBC was associated with an average increase in costs of £5407 (95% CI, 2663-8152).
Conclusion
Evidence from the BOXIT trial suggests that patients with NMIBC will both experience decrements in HRQoL and incur significant costs, especially in the event of a grade 3 recurrence or a progression to MIBC.
Keywords: Cost, HRQoL, NMIBC, RCT, QALY
Micro-Abstract
Limited evidence exists regarding the costs and health-related quality of life (HRQoL) effects of bladder cancer. Our study derived both the mean and marginal UK HRQoL and cost effects across multiple grades and stages of bladder cancer using data from the BOXIT (bladder COX-2 inhibition trial; n = 472). We found that patients with bladder cancer will experience decrements in HRQoL, which impose significant costs in the event of disease recurrence or progression that increase with the abnormality and invasiveness of the lesion. The results of the present study will help to lay the foundation for future burden of disease studies and cost-effectiveness analyses.
Introduction
Bladder cancer is the 9th most common cancer and ranks 13th in terms of cancer-associated mortality worldwide.1 In the United Kingdom, bladder cancer accounts for 3% of all new cancer cases, with an estimated 10,171 new cases diagnosed in 2015.2 Clinically, the lesions will be stratified using the TMN classification, with non–muscle-invasive bladder cancer (NMIBC) classified as stage Tis, Ta, and T1 and muscle-invasive bladder cancer (MIBC) as stage T2, T3, and T4. This distinction is important because the involvement of cancer invading the muscle carries a significantly worse prognosis and requires radical cystectomy, radical chemotherapy, or radical radiotherapy, with or without neoadjuvant chemotherapy. NMIBC has had more favorable survival rates but recurs frequently and has been associated with repeated outpatient visits, cytologic and cystoscopic monitoring, and adjuvant intravesical treatment regimens after transurethral resection.
In the European Union, it has been estimated that the treatment of bladder cancer costs €4.9 billion, representing 5% of the total healthcare cancer costs.3 In the United States, bladder cancer has been the most costly cancer to treat on a per patient basis.4,5 Having estimates of the cost and health-related quality of life (HRQoL) effects of clinical events related to bladder cancer is important as a means of understanding its burden, informing resource allocation decisions, and aiding further research. However, current evidence on such effects has been limited in several ways. First, the distinction between NMIBC recurrence and progression to MIBC has often been overlooked.5, 6, 7, 8 Second, HRQoL studies have predominantly focused on treatment-specific effects6, 7, 8, 9 and have not sought to understand the HRQoL effects of specific clinical events such as recurrence and progression. Third, systematic reviews have repeatedly criticized the internal validity of HRQoL analyses, commonly citing the use of retrospective or cross-sectional designs, nonvalidated instruments, short time horizons, and failure to adjust for confounders.7, 8, 9, 10, 11 Finally, a paucity of UK-specific cost analyses have been reported.
The present study aimed to estimate the expected costs and HRQoL of patients with a diagnosis of NMIBC and evaluate the effects associated with NMIBC recurrence and progression to MIBC. Our study used evidence from a recent randomized controlled trial of patients with intermediate- and high-risk bladder cancer, the BOXIT trial (bladder COX-2 [cyclo-oxygenase-2] inhibition trial).
Materials and Methods
BOXIT Trial
The BOXIT trial (ISRCTN registry no. ISRCTN84681538; Cancer Research UK no. CRUK/07/004) was a randomized phase III placebo-controlled trial that evaluated the addition of celecoxib to standard treatment for patients with NMIBC and an intermediate or a high risk of recurrence. From 2007 to 2012, 472 patients with transitional cell carcinoma NMIBC were recruited. The patients had a mean age of 65.9 years, and most patients were men (79%). The median follow-up at the point of analysis was 44 months (interquartile range, 36-57 months). The trial found no clear treatment benefit for celecoxib, with no significant differences in the interval to the first recurrence of bladder cancer (ie, NMIBC or MIBC) between patients randomized to either celecoxib or placebo for 2 years. Further details of the study design, treatment schedules, patients, and clinical results from the trial have been previously reported.12
Clinical Events
At trial entry, cases of intermediate- and high-risk NMIBC were defined according to the clinicopathologic features outlined by the European Association of Urology 2002 guidelines.13 The clinical events of interest during the trial were NMIBC recurrence and progression to MIBC. The grade and stage of NMIBC and MIBC were classified using the World Health Organization TNM classification.14 Patients could have experienced > 1 recurrence of NMIBC during the follow-up period. Disease progression was defined as the development of MIBC (stage ≥ pT2). Intermediate- and high-risk patients were recommended to undergo single adjuvant intravesical mitomycin C. The intermediate-risk patients were recommended to undergo 6 cycles of once-weekly adjuvant intravesical mitomycin C, and high-risk patients were recommended to undergo induction bacillus Calmette-Guérin with maintenance therapy for 3 years in accordance with international guidelines.15,16 Surveillance cystoscopy was performed at 3-month intervals for the first 2 years and then every 6 months for the third and fourth years. In the present report, we focused on the first 3 years of follow-up.
HRQoL, Resource Use, and Cost Data
HRQoL was measured using the EQ-5D-3L, a generic, preference-based measure encompassing 5 dimensions of health (ie, mobility, self-care, usual activities, pain or discomfort, anxiety or depression) and an overall health rating, measured using a visual analog scale.17 The HRQoL values were generated using reported UK preference “tariffs” for the 243 health states described in the EQ-5D-3L.18 The values range from 1.0 (perfect health status) to −0.594, with 0 indicating death and negative values reflecting health states considered to be worse than death.19 The 346 high-risk patients in the trial completed scheduled EQ-5D self-assessments at baseline (trial entry) and at 2, 3, 6, 12, 24, and 36 months of follow-up. The 126 intermediate-risk patients completed scheduled EQ-5D self-assessments at baseline and at 12, 24, and 36 months of follow-up.
The cost analysis used resource use data from questionnaires collected during the trial and took the perspective of the National Health Service and personal social services. The relevant resources used were those related to the diagnosis, treatment, and 3-year follow-up data of the patients included in the BOXIT trial. These included endoscopic investigations, together with the primary, secondary, and palliative care data, and the therapeutic procedures used, including radical cystectomy, chemotherapy, radical radiotherapy, immunotherapy regimens, and intravesical therapy regimens. Missing information relating to the quantity or specific type of treatment administered after a clinical event was assumed to follow usual practice. The unit costs were obtained from a variety of sources (Supplemental Table 1 available in the online version) and inflated to 2017 prices.20 The costs of inpatient visits were determined using a fixed component relating to the first 2 days of stay, with a marginal component related to any additional days. Care was assumed to have been elective, unless stated otherwise. The total costs were aggregated into years after baseline, with each year estimated by multiplying the number of resources consumed during that period by their respective unit costs and summating.
The HRQoL analysis set consisted of the 316 high-risk patients who had fully completed ≥ 1 EQ-5D questionnaire(s) during the trial. The focus on high-risk patients was to use the most EQ-5D data available and provide the most interpretable estimates of effects, given the small number of MIBC and grade 3 NMIBC events in the intermediate-risk patients and the different EQ-5D follow-up schedules for the 2 risk groups. An analysis that included both risk groups with annual EQ-5D follow-up data was performed as a secondary analysis.
Analysis Methods
The standard approach used to analyze HRQoL and cost data from clinical trials has been to compare these between treatment arms over time to calculate the quality-adjusted life-years (QALYs) and total costs for each patient in the trial.21 For trials showing no clinically or statistically significant benefit from a new treatment, this method will have little value. However, such trials offer a means of estimating the costs and HRQoL associated with a disease. This can include an exploration of how the HRQoL and costs vary between patients and how the patient characteristics and the clinical events they experience could explain some of this variation.22,23 Such analyses can provide valuable information to those assessing the potential value of other new treatments for similar patients.24
In the present study, 2 forms of analysis were conducted for both costs and HRQoL. The first analysis was descriptive, with the mean EQ-5D scores calculated at each follow-up period of interest, and the mean costs calculated annually. The patients were grouped in accordance with the types of events experienced during the 3-year follow-up period. The costs were categorized into resource-related groups for comparison. The second analysis was used to establish the effects of an event (ie, NMIBC, MIBC) on each outcome measure. The patients’ clinical events were linked to their closest post-event assessment. If multiple NMIBC recurrences had occurred between the EQ-5D or cost assessments, the recurrence with the highest grade was recorded. The effects of the events on the HRQoL and costs were computed using repeated measures regression, controlling for the relevant baseline covariates chosen on the basis of clinical relevance. These included: baseline HRQoL, randomized treatment, history of bladder cancer, patient characteristics (ie, age, body mass index, gender, diabetes), follow-up year, risk group, and interaction terms, as appropriate.
To evaluate the HRQoL and costs, separate generalized estimating equation models were implemented in accordance with reporting guidelines.25,26 The model fit, comparison, and selection of the working correlation structure was performed using the quasi-likelihood information criterion.27,28 Dependent variables of the annual costs and EQ-5D scores were assumed to follow the gamma and normal distributions, respectively.
Results
Patient Characteristics and Events
Patients experiencing disease recurrence and progression had characteristics similar to those of the patients without disease recurrence and progression. However, modest differences in the rates of diabetes and a history of NMIBC were noted (Table 1). We assessed whether systematic differences were present between patients with and without missing EQ-5D data at different follow-up points and found that the differences were small (Supplemental Tables 2 and 3 available in the online version). This finding supported the assumption from our complete case analysis that the occurrence of missing data was completely at random.
Table 1.
Patient Characteristics
| Characteristic | Total (n = 472) | High Risk (n = 346) | Intermediate Risk (n = 126) | No Event (n = 321) | Progression (n = 29) | Recurrencea |
|||
|---|---|---|---|---|---|---|---|---|---|
| Overall (n = 138) | Grade 1 (n = 36) | Grade 2 (n = 62) | Grade 3 (n = 46) | ||||||
| EQ-5D, baseline | 0.87 ± 0.15 | 0.86 ± 0.17 | 0.85 ± 0.22 | 0.88 ± 0.15 | 0.87 ± 0.13 | 0.87 ± 0.16 | 0.85 ± 0.20 | 0.91 ± 0.11 | 0.87 ± 0.14 |
| Age, y | 65.9 ± 9.9 | 65.8 ± 10.3 | 66.2 ± 8.8 | 65.7 ± 10.2 | 67.8 ± 7.1 | 66.2 ± 9.3 | 65.9 ± 10.3 | 66.1 ± 7.8 | 68.0 ± 7.7 |
| BMI, kg/m2 | 27.8 ± 4.6 | 27.9 ± 4.6 | 27.7 ± 4.5 | 27.8 ± 4.3 | 27.0 ± 4.2 | 28.1 ± 5.2 | 27.8 ± 6.5 | 28.7 ± 5.5 | 27.9 ± 4.6 |
| Male gender | 374 (79.2) | 278 (80.3) | 96 (76.2) | 262 (81.6) | 25 (86.2) | 102 (73.9) | 27 (75.0) | 45 (72.6) | 33 (71.7) |
| Diabetes | 42 (8.9) | 30 (8.7) | 12 (9.6) | 23 (7.2) | 2 (6.9) | 19 (13.8) | 6 (16.7) | 8 (12.9) | 8 (17.4) |
| NMIBC history | 159 (34.0) | 95 (27.8) | 64 (51.2) | 94 (29.7) | 14 (48.3) | 58 (42.3) | 17 (47.2) | 30 (48.4) | 16 (35.6) |
| Celecoxib | 236 (50.0) | 167 (48.3) | 69 (54.8) | 164 (51.1) | 13 (44.8) | 65 (47.1) | 22 (61.1) | 30 (48.4) | 17 (37.0) |
| Smoking status | |||||||||
| Never | 145 (39.6) | 113 (33.0) | 32 (25.8) | 101 (31.8) | 8 (28.6) | 42 (30.9) | 10 (2.8) | 16 (26.2) | 18 (40.0) |
| Previous | 252 (54.1) | 187 (54.7) | 65 (52.4) | 173 (54.4) | 16 (57.1) | 70 (51.5) | 19 (52.8) | 34 (55.7) | 21 (46.7) |
| Current | 69 (14.8) | 42 (12.3) | 27 (21.8) | 44 (13.8) | 4 (14.3) | 24 (17.7) | 7 (19.4) | 11 (18.0) | 6 (13.3) |
| ECG result | |||||||||
| Normal | 370 (78.6) | 276 (79.8) | 94 (75.2) | 250 (78.1) | 24 (82.8) | 109 (79.0) | 8 (77.8) | 49 (79.0) | 37 (80.4) |
| Abnormal | 101 (21.4) | 70 (20.2) | 31 (24.8) | 70 (21.9) | 5 (17.2) | 29 (21.0) | 28 (77.8) | 13 (20.1) | 9 (19.6) |
Data presented as mean ± standard deviation or n (%).
Abbreviations: ECG = electrocardiogram; NMIBC = non–muscle-invasive bladder cancer.
The number of patients experiencing a recurrence exceeded the sum of graded recurrences because of missing grade data and patients experiencing multiple recurrences of different grades.
The recurrence of NMIBC was > 8 times more common than was progression to MIBC. A total of 233 cases of NMIBC recurrence in 138 patients (29.2%; of all 472 patients) had been recorded during the 3-year follow-up period. In contrast, 29 patients (6.1%) had experienced progression to MIBC (62.1% had undergone subsequent radical surgery). Of the 233 recurrent NMIBC events, 37 were not graded, 46 (9.7%) had experienced ≥ 1 grade 3 NMIBC recurrence (32.6% had undergone subsequent radical surgery), and 62 (13.1%) and 36 (7.6%) patients, respectively, had experienced ≥ 1 grade 2 and grade 1 recurrences (jointly, 4.1% had undergone subsequent radical surgery). Further details of the clinical events in the trial are provided in Supplemental Table 4 (available in the online version).
HRQoL Analysis
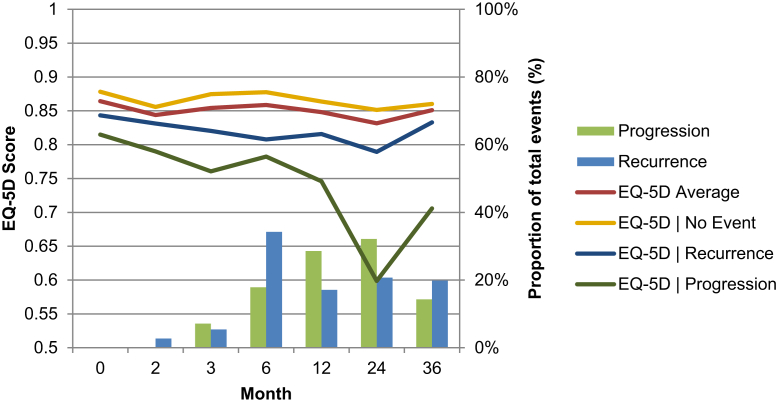
The completion rate of the EQ-5D during the 3-year period was 79% (range, 58%-84%) across the points of follow-up. The completion rates after a NMIBC recurrence and progression to MIBC were 60% and 38%, respectively. An overview of the observed mean EQ-5D index scores for high-risk patients and the proportion of events occurring between each EQ-5D follow-up period are presented in Figure 1. Full details of the HRQoL descriptive results are provided in Supplemental Tables 5 and 6 (available in the online version).
Figure 1.
EQ-5D Scores for High-risk Patients for Each Event-related Subgroup and Associated Proportion of Events in Each Follow-up Point During 3 Years of Follow-up. The x-Axis Represents Time in Months After Baseline With Categories and Their Distance Solely Indicative of Trial Follow-up and Not Equating to the Length of Time Between Intervals
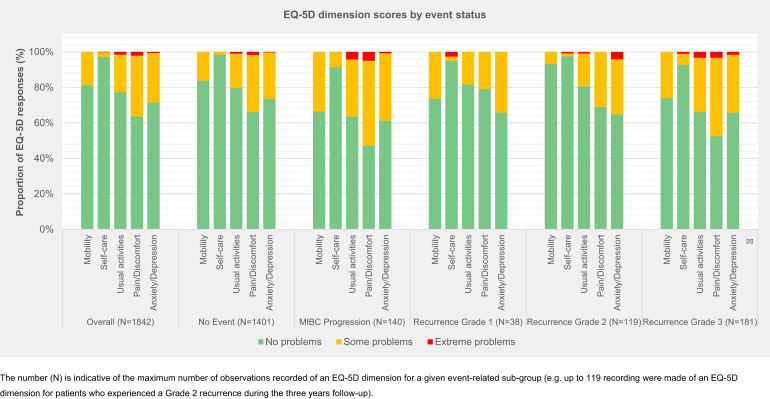
The set of subgroups comprising patients who had experienced ≥ 1 of the specified clinical events during the 3-year follow-up period or had experienced no event is presented in Figure 1. These findings suggest that NMIBC recurrence and MIBC progression could be associated with deterioration in HRQoL at specific points. The variation in HRQoL at specific follow-up points was largely driven by the events experienced by the patients. In contrast, the variation in HRQoL between the follow-up points was related to the underlying within-patient variations, the nonuniform distribution of events over time, and the sampling error, exacerbated by partitioning modestly sized subgroups. A comparison of the EQ-5D dimensions stratified by the event-related subgroup found greater proportions of patients reporting problems with pain or discomfort and undertaking usual activities when experiencing a grade 3 recurrence or MIBC progression compared with no events during the 3-year follow-up period (Supplemental Figure 1 available in the online version).
Supplemental Figure 1.
EQ-5D Responses Stratified by Dimension and Severity Level for High-risk Patients for Each Event-related Subgroup During 3 Years of Follow-up. The Number Indicates the Maximum Number of Observations Recorded of an EQ-5D Dimension for a Given Event-related Subgroup (eg, ≤ 119 Recordings Were Made of an EQ-5D Dimension for Patients Who Had Experienced a Grade 2 Recurrence During the 3-year Follow-up Period)
The statistically significant effects of clinical events on HRQoL in terms of the estimated decrements and mean health-state values are listed in Table 2. Progression to MIBC and NMIBC grade 3 recurrence were associated with predicted mean decrements in HRQoL of −0.10 (95% confidence interval, −0.17 to −0.03) and −0.08 (95% confidence interval, −0.13 to −0.03), respectively (P < .01). In contrast, the recurrence of NMIBC grade 1 and grade 2 was associated with positive, but statistically insignificant (P > .1), increments in HRQoL compared with patients without cancer.
Table 2.
Estimated Statistically Significant Effects on HRQoL and Associated Health State Values From Clinical Events (High-risk Patients Only)
| Variable | Estimated HRQoL Decrementa | Estimated Health State Valuea |
|---|---|---|
| No event | NA | 0.84606 (0.83292 to 0.85921) |
| NMIBC recurrence (grade 3) | −0.08306b (−0.13379 to −0.03233) | 0.76300 (0.71178 to 0.81422) |
| MIBC progression | −0.09909b (−0.17256 to −0.02561) | 0.74698 (0.67309 to 0.82087) |
Data presented as mean (95% confidence interval).
Abbreviations: HRQoL = health-related quality of life; MIBC = muscle-invasive bladder cancer; NA = not applicable; NMIBC = non–muscle-invasive bladder cancer.
Multivariate HRQoL longitudinal model controlled for baseline EQ-5D score, treatment (celecoxib), patient characteristics, bladder cancer history, annual time dummies, and events.
P < .01.
The secondary analysis showed that introducing an interaction term into the regression analysis revealed that patients with grade 3 NMIBC recurrence in the first year experienced larger decrements in HRQoL (−0.11) compared with those with recurrence in subsequent years (−0.04). The small numbers precluded the same analysis for MIBC progression. Including both high- and intermediate-risk patients in the analysis using only the annual EQ-5D assessment data generated NMIBC recurrence estimates closer to 0 for all grades, with only MIBC events resulting in a statistically significant decrement in HRQoL (P < .05). Irrespective of the bladder cancer grade or stage, radical cystectomy was associated with a −0.17 decrement in HRQoL. All regression results and primary variance–covariance matrices are presented in Supplemental Table 10, Supplemental Table 11, Supplemental Table 12, Supplemental Table 13, Supplemental Table 7, Supplemental Table 8, Supplemental Table 9 (available in the online version).
Cost Analysis
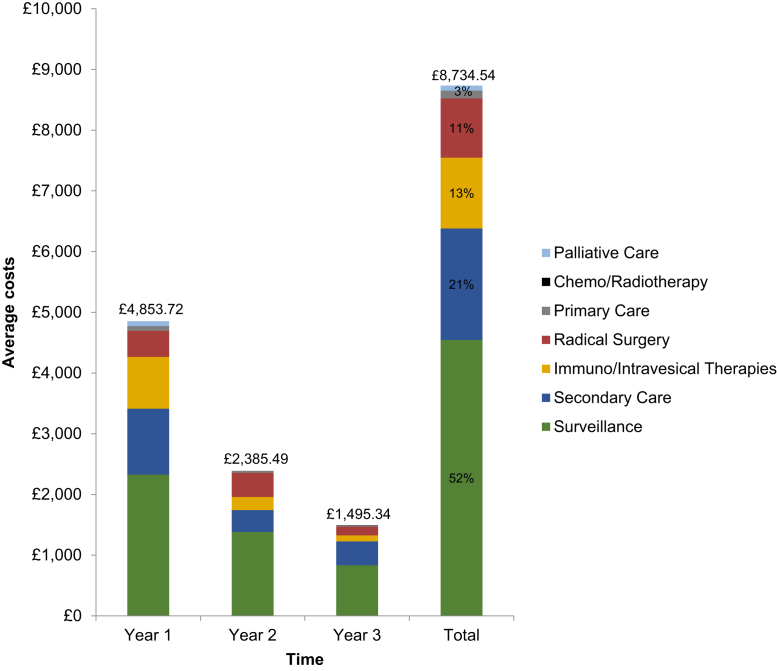
The mean costs per patient for each type of care (Supplemental Table 1 available in the online version), annually and in total, are reported in Figure 2. The mean cost of treatment for a patient with NMIBC was £4854 in the first year, with a total cost of £8735 over three years. These results suggest that the costs decline over time, with mean costs of £1496 in year 3. Endoscopic surveillance was the principal cost driver, accounting for > 52% of the total costs and representing a high proportion in years 2 (£1384 of £2386) and 3 (£835 of £1496). These estimates resulted in a 3-year total cost for the UK NMIBC bladder cancer cohort diagnosed in 2015 at ~£66.14 million, assuming that 74.5% of the 10,171 UK bladder cancer cases were NMIBC.2,29
Figure 2.
Mean Costs per Patient Over Time Stratified by Resource Category for Intermediate- and High-risk Patients
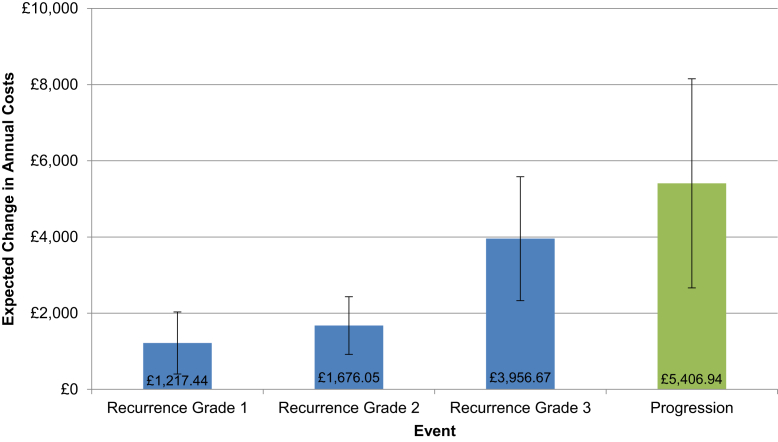
The effect of the clinical events on annual costs is shown in Figure 3, which indicated that MIBC progression and all grades of NMIBC recurrence led to increased costs. Higher grades of NMIBC were associated with higher costs, with grade 3 recurrence events necessitating more intensive therapy and closer surveillance. Progression to MIBC was associated with the greatest cost increment, with a £5407 increase in the expected annual cost per patient, again reflecting the more intensive therapy. Additionally, the treatment and surveillance of high-risk patients were associated with a £1968 increase in mean costs in the first year, although the costs had declined to £457 and £74 in years 2 and 3, respectively. The predicted mean costs per patient by year, event status, and risk group are shown in Table 3.
Figure 3.
Estimated Mean Change in Annual Cost per Patient Associated With Clinical Events (95% Confidence Intervals Shown by Vertical Bars) From a Multivariate Longitudinal Panel Cost-related Analysis Controlling for Treatment, Patient Characteristics, Risk Group, Annual Time Dummies, Bladder Cancer Events, and Interactions
Table 3.
Estimated Patient Costs Across Time, Risk Group, and Event Statusa
| Risk Group | Year | No Bladder Cancer | NMIBC Recurrence |
MIBC Progression | ||
|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | ||||
| High | ||||||
| 1 | £4796 | £6014 | £6472 | £8753 | £10,374 | |
| 2 | £2363 | £3581 | £4039 | £6320 | £7940 | |
| 3 | £1387 | £2605 | £3063 | £5344 | £6964 | |
| Intermediate | ||||||
| 1 | £2828 | £4046 | £4505 | £6785 | £8406 | |
| 2 | £1907 | £3125 | £3583 | £5864 | £7484 | |
| 3 | £1314 | £2532 | £2990 | £5271 | £6891 | |
Abbreviations: HRQoL = health-related quality of life; MIBC = muscle-invasive bladder cancer; NA = not applicable; NMIBC = non–muscle-invasive bladder cancer.
Predicted values from a multivariate longitudinal panel cost-related analysis controlling for treatment, patient characteristics, risk group, annual time dummies, bladder cancer events, and interactions.
Discussion
Published economic evaluations of treatments for bladder cancer have lacked robust estimates of clinical effects on HRQoL and costs.30,31 Furthermore, clinicians should understand the consequences of clinical events on patients’ well-being and the health service costs. The present study has provided new evidence on the costs and HRQoL associated with NMIBC occurrence, recurrence, and progression to MIBC, supporting future clinical and economic evaluations. Our findings suggest that NMIBC will have an average cost of £8735 during a 3-year period, with cases of grade 1, 2, and 3 NMIBC recurrences and progression to MIBC associated with £1218, £1677, £3957, and £5407 increases in annual costs, respectively. In addition, grade 3 recurrence and progression to MIBC were associated with statistically significant decrements in HRQoL (−0.08 and −0.10, respectively).
Singer et al32 reported that patients with bladder cancer, whether muscle invasive or not, will experience significant and clinically relevant deteriorations in HRQoL. Little evidence has contradicted the idea that patients with MIBC will experience a significant health burden; however, the same cannot necessarily be said for those with NMIBC. The commonly reported NMIBC morbidities have included mental health effects at diagnosis, physical discomfort, sexual problems, and urinary symptoms.33, 34, 35 However, these have rarely translated into reductions in longer term health outcomes and, in some cases, have not been recorded at all.9,36 It has been suggested that patients might become “accustomed” to NMIBC and its related management, accepting recurrences as a part of their lives.10 The evidence presented from the BOXIT trial has offered some additional support for this view, but suggests that not all cases of NMBIC recurrence should be considered equal. Based on the recommended NMIBC surveillance guidelines, our results suggest that the negative effect of a NMIBC recurrence on HRQoL will be concentrated within the high-grade strata (grade 3), especially in the first year after the diagnosis. Furthermore, no evidence of negative HRQoL outcomes from grade 1 or 2 NMIBC recurrences was found. This might, at least in part, be explained by the low rates of radical surgery observed for grade 1 and 2 NMIBC recurrences. The results from supplementary analyses have supported these findings, with the use of cystectomy a large and significant predictor of HRQoL status. In addition, the patient groups with the highest rates of radical surgery (for grade 3 recurrence and progression) were most likely to report related problems with pain or discomfort and undertaking usual activities. A fuller understanding of the mechanisms behind these findings requires further prospective research.
Sangar et al,37 estimated that the UK cost in 2001 to 2002 for the diagnosis, treatment, and 5-year follow-up of each bladder cancer case was £55.39 million, at a mean cost of £8349.20. Allowing for inflation and the different follow-up periods, their results are similar to those from the present study. To put this into context, it would be less costly per patient to treat stage 2 colon, rectal, and non–small-cell lung cancer in the United Kingdom.38 The results from our analysis compliment those from the earlier study, showing the prominent role of endoscopic surveillance in driving the costs, which has remained the primary target for innovation in bladder cancer management.5,39,40 Optimizing surveillance has also remained a research priority. Less costly and noninvasive urinary biomarkers represent an attractive option; however, to date, no commercially available test has the diagnostic accuracy to replace cystoscopy because patients and physicians require a test with high sensitivity before widespread acceptance.41, 42, 43 Similar to others, we found that progression to MIBC will be associated with higher costs for intermediate- and high-risk patients.44
The relatively large sample size, prospective study design, and the use of a validated HRQoL instrument represent the strengths of the present study. To the best of our knowledge, this is the first study to estimate both the mean and the marginal HRQoL and the cost effects across multiple grades and stages of bladder cancer. However, the present study had several important limitations. Despite the BOXIT protocol remaining representative of current UK guidelines (other than celecoxib treatment), differences between the BOXIT trial and current clinical practice have occurred (eg, the European Association of Urology now recommends bacillus Calmette-Guérin instillations for intermediate-risk patients and have revised the definitions of risk45). In addition, the trial’s exclusion criteria could have limited the generalizability of our study, with results applicable to a cohort healthier than what might be observed in clinical practice. With respect to HRQoL, the EQ-5D is a generic measure of health outcomes suitable to assess the value of healthcare interventions across different disease areas. The EQ-5D is the preferred instrument of the National Institute for Health and Care Excellence for cost-effectiveness analysis. Although the measure showed important differences between the patient subgroups, further research could assess whether the EQ-5D is sufficiently sensitive to detect important clinical changes in patients with bladder cancer. Regarding the study findings, the true negative repercussions of MIBC might differ from those reported because the number of patients who progressed to MIBC was relatively small because BOXIT trial was powered to investigate the interval to the first recurrence. This, coupled with the low postprogression EQ-5D response rate, resulted in uncertain estimates and might lead to overestimates of the HRQoL because patients with relatively poor health outcomes after the development of MIBC might be less likely to complete the EQ-5D. Moreover, the increasingly protracted EQ-5D follow-up periods meant that the clinical events in the study became progressively distant from completion of the EQ-5D. Whether improvements in the reported postevent HRQoL outcomes over time stemmed from the true underlying dynamics of bladder cancer or had resulted only from time-related disparities between the event and the follow-up evaluation remains to be determined.
The costs could have been underestimated for several reasons. First, our analysis of the effect of the events on the annual costs neglected the potential dynamics and spillover effects between the evaluation periods. Bladder cancer events will inevitably prompt immediate resource use; however, the costs incurred from stricter surveillance and the greater risk of related events will be realized further into the future. Understanding these dynamics requires a more detailed collection of the resource use data and remains a potential avenue for further research. Second, the assumption that the treatments were elective could, again, have underrepresented the costs. Third, the protracted and persistent nature of bladder cancer has far broader cost effects than those incurred only by the NHS within 3 years. A wider perspective would give a more comprehensive account of the earnings, productivity, and time lost by patients with bladder cancer and their informal caregivers.
Conclusion
The results from our analysis of the BOXIT trial data suggest that patients with NMIBC will experience decrements in HRQoL, with significant costs imposed in the event of disease recurrence or progression, and the costs increasing with the abnormality and invasiveness of the lesion.
Clinical Practice Points
-
•
A need exists to evaluate the costs and HRQoL implications of bladder cancer and its recurrence and progression to assess the disease burden, inform resource allocation decisions, and aid further research.
-
•
It has been shown that NMIBC will be associated with considerable costs in the United Kingdom and that patients will experience significant decrements in HRQoL with progression to MIBC; however, the effect from NMIBC recurrence is less clear.
-
•
In our study, we reported both the mean and the marginal UK HRQoL and cost effects across multiple grades and stages of bladder cancer for patients with NMIBC and found significant decrements in HRQoL related to grade 3 recurrence and progression to MIBC; the cost effects increased with the lesion's abnormality and invasiveness.
-
•
The evidence presented from the BOXIT trial suggests that not all cases of NMBIC recurrence should be considered equal with respect to the effects on patient HRQoL or the consequent healthcare costs.
-
•
The results from the present study could help to lay the foundation for future related burden of disease studies and cost-effectiveness analyses.
Disclosure
E.H. reports grants from Cancer Research UK, grants from Kyowa Hakko UK, grants from Alliance Pharma (previously Cambridge Laboratories Ltd), nonfinancial support from Pfizer Inc, during the conduct of the study, grants from Pfizer Inc, grants and nonfinancial support from Merck Sharp & Dohm, grants and nonfinancial support from Astra Zeneca, grants from Janssen-Cilag, grants and nonfinancial support from Bayer, grants from Aventis Pharma Ltd (Sanofi), and grants from Accuray Inc. M.S. reports grants from Cancer Research UK (CRUK/07/04) and educational grants from Kyowa Hakko UK Ltd and Cambridge Laboratories Ltd during the conduct of the study. The remaining authors declare that they have no competing interests.
Acknowledgments
Grateful thanks are due to the BOXIT Management Group and Trial Steering Committee for the release of data to conduct this research. Thanks also to all the patients who participated in the study; all involved staff at the participating centers and contributing principal investigators; and the trial unit staff at Institute of Cancer Research-Clinical Trials and Statistics Unit involved in the coordination, data management, and analysis of the trial data. The BOXIT was supported by Cancer Research UK (CRUK 07/004). Pfizer US provided the celecoxib and placebo drugs, and education grants were received from Kyowa Hakko UK Limited and Alliance Pharma Plc (formerly, Cambridge Laboratories Ltd). Trial recruitment and on-going patient follow-up were supported within centers by the National Institute for Health Research funded National Cancer Research Network. Central trial management costs were funded by Cancer Research UK (grant C8262/A5669). None of the funders played a role in the study design, data collection, analysis, interpretation, writing of the report, or the decision to submit the report for publication.
Footnotes
Supplemental figure and tables accompanying this article can be found in the online version at https://doi.org/10.1016/j.clgc.2019.12.004.
Supplemental Data
Supplemental Table 1.
Unit Costs
| Care | Unit Costsa | Source |
|---|---|---|
| Primary care | PSSRU health and social care, 201720 | |
| GP home visit | £86 | |
| Specialist nurse home visit | £57 | |
| General practice surgery visit, GP | £32 | |
| General practice surgery visit (nurse) | £10 | |
| Secondary care | NHS schedule reference costs 2016-201746 | |
| Outpatient attendance | £108 | TOA: urology outpatient attendance (service code, 101) |
| Inpatient attendance | £820 | EL: minor bladder procedures, age ≥ 19 y (HRG code, LB15E) |
| Inpatient excess days | £397 | EL XS: intermediate open bladder procedures (HRG code, LB12Z) |
| Palliative careb | £12,968 | NICE technology assessment January 201047 |
| Surveillance | NICE technology assessment January 201047 | |
| Flexible cystoscopy | £449 | |
| Rigid cystoscopy | £1176 | |
| Intravesical/immunotherapy | ||
| Mitomycin instillation | £80 | British National Formulary 2018 |
| Bacillus Calmette-Guérin instillation | £101 | NICE technology assessment January 201047 |
| Radical surgery | ||
| Cystectomy | £9973 | Total HRGs: cystectomy with urinary diversion and reconstruction (code, LB39C/LB39D) |
| Lobectomy | £6601 | NICE clinical guideline 121 (2011)48 |
| Nephroureterectomy | £6471 | Complex, open or laparoscopic, kidney or ureter procedures, with CC score 0-1 (HRG code, LB60F) |
| Renogram | £256 | Renogram, age ≥ 19 y (HRG code, RN25A) |
| Chemotherapy/radiotherapyc | ||
| Radical radiotherapy | £1156 | NICE technology assessment January 201047 |
| Gemcitabine, cisplatin | £169 | eMit drug unit costs and London Cancer Network administration schedules |
| Gemcitabine, carboplatin | £232 | eMit drug unit costs and London Cancer Network administration schedules |
| 5-FU, MMC | £104 | eMit drug unit costs and London Cancer Network administration schedules |
| Carboplatin, etoposide | £173 | eMit drug unit costs and London Cancer Network administration schedules |
Abbreviations: 5-FU = 5-fluorouracil; CC = complexity and comorbidity; EL = elective inpatient; EL XS = elective inpatient excess bed days; eMIT = electronic market information tool; GP = general practitioner; HRGs = healthcare resource groups; HRQoL = health-related quality of life; MIBC = muscle-invasive bladder cancer; MMC = mitomycin; NA = not applicable; NHS = National Health Service; NICE = National Institute for Health and Care Excellence; NMIBC = non–muscle-invasive bladder cancer; PSSRU = Personal Social Service Research Unit; TOA = total outpatient attendance.
Inflated to 2017 prices using the PSSRU hospital and community health services index; presented costs were rounded up to the nearest pound sterling.
Duration of 135 days used in accordance with reference material and per day NHS schedule reference for 2016-2017 costs applied.
Specific chemotherapy unit costs were calculated as the product of the specific drug costs (using eMit), dosage, and observed/recommended number of cycles (recommended schedules from the NHS Cancer Network were used if trial information was missing).
Supplemental Table 2.
Summary Statistics Comparison: Missing and Nonmissing EQ-5D Data Collectiona
| Variable | Missing Values |
Nonmissing Values |
||
|---|---|---|---|---|
| n | Mean | n | Mean | |
| Month 2 | ||||
| Age | 187 | 65.19 | 285 | 66.38 |
| BMI | 180 | 27.71 | 266 | 27.89 |
| Gender | 187 | 75% | 285 | 82% |
| Never smoked | 48 | 26% | 97 | 34% |
| Previous smoker | 97 | 53% | 155 | 55% |
| Current smoker | 39 | 21% | 30 | 11% |
| ECG result | 186 | 23% | 285 | 21% |
| Celecoxib | 187 | 52% | 285 | 48% |
| Diabetes | 186 | 11% | 285 | 8% |
| History | 183 | 44% | 284 | 27% |
| Month 3 | ||||
| Age | 185 | 65.41 | 287 | 66.26 |
| BMI | 179 | 27.77 | 267 | 27.85 |
| Gender | 185 | 75% | 287 | 82% |
| Never smoked | 44 | 24% | 101 | 35% |
| Previous smoker | 97 | 53% | 155 | 55% |
| Current smoker | 41 | 23% | 28 | 10% |
| ECG result | 184 | 21% | 287 | 22% |
| Celecoxib | 185 | 54% | 287 | 47% |
| Diabetes | 184 | 11% | 287 | 8% |
| History | 181 | 46% | 286 | 27% |
| Month 6 | ||||
| Age | 196 | 65.27 | 276 | 66.40 |
| BMI | 189 | 27.66 | 257 | 27.93 |
| Gender | 196 | 74% | 276 | 83% |
| Never smoked | 50 | 26% | 95 | 35% |
| Previous smoker | 103 | 53% | 149 | 55% |
| Current smoker | 40 | 21% | 29 | 10% |
| ECG result | 195 | 21% | 276 | 22% |
| Celecoxib | 196 | 53% | 276 | 48% |
| Diabetes | 195 | 12% | 276 | 7% |
| History | 192 | 46% | 275 | 26% |
| Month 12 | ||||
| Age | 125 | 65.67 | 347 | 66.02 |
| BMI | 120 | 27.91 | 326 | 27.78 |
| Gender | 125 | 78% | 347 | 80% |
| Never smoked | 31 | 25% | 114 | 33% |
| Previous smoker | 66 | 54% | 186 | 54% |
| Current smoker | 25 | 21% | 44 | 13% |
| ECG result | 124 | 19% | 347 | 22% |
| Celecoxib | 125 | 52% | 347 | 49% |
| Diabetes | 124 | 9% | 347 | 9% |
| History | 122 | 34% | 345 | 34% |
| Month 24 | ||||
| Age | 163 | 66% | 309 | 66.03 |
| BMI | 155 | 27.65 | 291 | 27.91 |
| Gender | 163 | 79% | 309 | 80% |
| Never smoked | 42 | 26% | 103 | 34% |
| Previous smoker | 90 | 56% | 162 | 53% |
| Current smoker | 28 | 18% | 41 | 13% |
| ECG result | 162 | 16% | 309 | 24% |
| Celecoxib | 163 | 52% | 309 | 49% |
| Diabetes | 162 | 10% | 309 | 8% |
| History | 160 | 36% | 307 | 33% |
| Month 36 | ||||
| Age | 191 | 66.21 | 281 | 65.73 |
| BMI | 183 | 27.62 | 263 | 27.95 |
| Gender | 191 | 79% | 281 | 79% |
| Never smoked | 47 | 25% | 98 | 35% |
| Previous smoker | 100 | 53% | 152 | 55% |
| Current smoker | 41 | 22% | 28 | 10% |
| ECG result | 190 | 20% | 281 | 22% |
| Celecoxib | 191 | 53% | 281 | 48% |
| Diabetes | 190 | 9% | 281 | 9% |
| History | 188 | 36% | 279 | 33% |
Abbreviations: BMI = body mass index; ECG = electrocardiography; EQ-5D = EuroQoL 5 dimension.
Patient gender (female = 0; male = 1); ECG result (normal = 1; abnormal = 0); Celecoxib (placebo arm = 0; treatment arm = 1); history (no history of NMIBC = 0; history of NMIBC = 1); diabetes (no diabetes = 0; diabetes = 1).
Supplemental Table 3.
Summary Statistics Comparison: Missing and Nonmissing Costsa
| Variable | Missing Values |
Nonmissing Values |
||
|---|---|---|---|---|
| n | Mean | n | Mean | |
| Year 1 | ||||
| Age | 25 | 69.16 | 447 | 65.75 |
| BMI | 23 | 27.29 | 423 | 27.85 |
| Gender | 25 | 80% | 447 | 79% |
| Never smoked | 6 | 24% | 145 | 32% |
| Previous smoker | 13 | 52% | 239 | 53% |
| Current smoker | 6 | 24% | 63 | 14% |
| ECG result | 24 | 8% | 447 | 22% |
| Celecoxib | 25 | 52% | 447 | 50% |
| Diabetes | 24 | 13% | 442 | 9% |
| History | 23 | 52% | 444 | 33% |
| Year 2 | ||||
| Age | 30 | 68.57 | 442 | 65.75 |
| BMI | 28 | 26.91 | 418 | 27.88 |
| Gender | 30 | 80% | 442 | 79% |
| Never smoked | 7 | 23% | 144 | 33% |
| Previous smoker | 19 | 63% | 223 | 53% |
| Current smoker | 4 | 13% | 65 | 15% |
| ECG result | 29 | 14% | 442 | 22% |
| Celecoxib | 30 | 47% | 442 | 50% |
| Diabetes | 29 | 10% | 442 | 9% |
| History | 28 | 39% | 439 | 34% |
| Year 3 | ||||
| Age | 47 | 68.09 | 425 | 65.69 |
| BMI | 45 | 27.15 | 401 | 27.89 |
| Gender | 47 | 74% | 425 | 80% |
| Never smoked | 11 | 23% | 140 | 33% |
| Previous smoker | 26 | 55% | 226 | 53% |
| Current smoker | 10 | 21% | 59 | 14% |
| ECG result | 46 | 11% | 425 | 23% |
| Celecoxib | 47 | 43% | 425 | 50% |
| Diabetes | 46 | 7% | 425 | 9% |
| History | 45 | 42% | 422 | 33% |
Abbreviations: BMI = body mass index; ECG = electrocardiography.
Patient gender (female = 0; male = 1); ECG result (normal = 1; abnormal = 0); Celecoxib (placebo arm = 0; treatment arm = 1); history (no history of NMIBC = 0; history of NMIBC = 1); diabetes (no diabetes = 0; diabetes = 1).
Supplemental Table 4.
Trial Eventsa
| Variable | Trial Event, n |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Month 2 |
Month 3 |
Month 6 |
Month 12 |
Month 24 |
Month 36 |
Total Events | Total Patients | |||||||
| HR | IR | HR | IR | HR | IR | HR | IR | HR | IR | HR | IR | |||
| MIBC progression | 0 | 0 | 2 | 0 | 5 | 0 | 8 | 0 | 9 | 1 | 4 | 0 | 29 | 29 |
| NMIBC recurrence | 3 | 2 | 6 | 6 | 38 | 20 | 19 | 32 | 23 | 44 | 22 | 18 | 233 | 138 |
| Recurrence grade | 2 | 2 | 6 | 4 | 35 | 14 | 16 | 29 | 17 | 37 | 17 | 17 | 196 | 121 |
| Unknown | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 3 | 3 |
| Grade 1 | 0 | 1 | 0 | 0 | 4 | 7 | 3 | 11 | 5 | 14 | 0 | 9 | 54 | 36 |
| Grade 2 | 1 | 0 | 3 | 4 | 9 | 7 | 4 | 15 | 7 | 21 | 7 | 7 | 85 | 62 |
| Grade 3 | 1 | 1 | 3 | 0 | 22 | 0 | 7 | 3 | 5 | 2 | 9 | 1 | 54 | 46 |
Abbreviations: EQ-5D = EuroQoL 5 dimension; HR = high-risk (patients); IR = intermediate-risk (patients); MIBC = muscle-invasive bladder cancer; NMIBC = non–muscle-invasive bladder cancer.
For cases in which multiple NMIBC recurrences had developed between the EQ-5D and/or annual cost assessments, the analysis set was applied to the recurrence with the highest grade recorded (see the “Materials and Methods” section for details).
Supplemental Table 5.
Observed EQ-5D Scores From the BOXIT Trial for High-risk Patients
| EQ-5D | EQ-5D Event-Specific Scores |
||||||
|---|---|---|---|---|---|---|---|
| Baseline | Month 2 | Month 3 | Month 6 | Month 12 | Month 24 | Month 36 | |
| Average | |||||||
| Mean ± SD | 0.86 ± 0.17 | 0.84 ± 0.20 | 0.85 ± 0.18 | 0.86 ± 0.18 | 0.85 ± 0.19 | 0.83 ± 0.19 | 0.85 ± 0.19 |
| Patients, n | 309 | 284 | 286 | 274 | 250 | 223 | 205 |
| No event | |||||||
| Mean ± SD | 0.88 ± 0.15 | 0.86 ± 0.20 | 0.87 ± 0.15 | 0.88 ± 0.16 | 0.86 ± 0.17 | 0.85 ± 0.16 | 0.86 ± 0.18 |
| Patients, n | 224 | 210 | 209 | 209 | 297 | 181 | 168 |
| Progression | |||||||
| Mean ± SD | 0.82 ± 0.23 | 0.79 ± 0.23 | 0.76 ± 0.22 | 0.78 ± 0.26 | 0.75 ± 0.26 | 0.60 ± 0.30 | 0.71 ± 0.35 |
| Patients, n | 28 | 26 | 28 | 19 | 15 | 10 | 7 |
| Recurrence | |||||||
| Mean ± SD | 0.84 ± 0.20 | 0.83 ± 0.20 | 0.82 ± 0.21 | 0.81 ± 0.21 | 0.82 ± 0.21 | 0.79 ± 0.25 | 0.83 ± 0.20 |
| Patients, n | 71 | 62 | 64 | 56 | 45 | 35 | 33 |
| Recurrence grade | |||||||
| 1 | |||||||
| Mean ± SD | 0.90 ± 0.11 | 0.85 ± 0.12 | 0.88 ± 0.14 | 0.86 ± 0.11 | 0.93 ± 0.20 | 0.81 ± 0.21 | 0.83 ± 0.33 |
| Patients, n | 8 | 7 | 8 | 7 | 4 | 4 | 4 |
| 2 | |||||||
| Mean ± SD | 0.88 ± 0.14 | 0.90 ± 0.10 | 0.89 ± 0.13 | 0.86 ± 0.20 | 0.84 ± 0.14 | 0.70 ± 0.32 | 0.78 ± 0.78 |
| Patients, n | 23 | 21 | 21 | 20 | 17 | 14 | 10 |
| 3 | |||||||
| Mean ± SD | 0.85 ± 0.17 | 0.82 ± 0.21 | 0.79 ± 0.23 | 0.75 ± 0.22 | 0.79 ± 0.26 | 0.77 ± 0.27 | 0.80 ± 0.22 |
| Patients, n | 36 | 31 | 33 | 27 | 20 | 16 | 6 |
Abbreviations: BOXIT = bladder COX-2 (cyclooxygenase-2) inhibition trial; EQ-5D = EuroQoL 5 dimension; SD = standard deviation.
Supplemental Table 6.
Observed EQ-5D Scores From the BOXIT Trial for Intermediate- and High-Risk Patients
| EQ-5D | EQ-5D Event-Specific Scores |
|||
|---|---|---|---|---|
| Baseline | Month 12 | Month 24 | Month 36 | |
| Average | ||||
| Mean ± SD | 0.86 ± 0.19 | 0.85 ± 0.20 | 0.83 ± 0.20 | 0.85 ± 0.20 |
| Patients, n | 410 | 347 | 309 | 281 |
| No event | ||||
| Mean ± SD | 0.87 ± 0.16 | 0.86 ± 0.18 | 0.84 ± 0.18 | 0.85 ± 0.20 |
| Patients, n | 275 | 244 | 224 | 209 |
| Progression | ||||
| Mean ± SD | 0.82 ± 0.23 | 0.71 ± 0.28 | 0.66 ± 0.55 | 0.71 ± 0.35 |
| Patients, n | 29 | 16 | 11 | 7 |
| Recurrence | ||||
| Mean ± SD | 0.85 ± 0.21 | 0.84 ± 0.23 | 0.84 ± 0.24 | 0.87 ± 0.19 |
| Patients, n | 121 | 95 | 78 | 68 |
| Recurrence grade | ||||
| 1 | ||||
| Mean ± SD | 0.81 ± 0.29 | 0.77 ± 0.31 | 0.80 ± 0.30 | 0.87 ± 0.26 |
| Patients, n | 28 | 24 | 21 | 18 |
| 2 | ||||
| Mean ± SD | 0.91 ± 0.12 | 0.88 ± 0.16 | 0.84 ± 0.26 | 0.88 ± 0.19 |
| Patients, n | 54 | 48 | 41 | 33 |
| 3 | ||||
| Mean ± SD | 0.86 ± 0.17 | 0.80 ± 0.27 | 0.77 ± 0.29 | 0.83 ± 0.21 |
| Patients, n | 41 | 26 | 21 | 20 |
Abbreviations: BOXIT = bladder COX-2 (cyclooxygenase-2) inhibition trial; EQ-5D = EuroQoL 5 dimension; SD = standard deviation.
Supplemental Table 7.
Primary HRQoL Regression
| Variable | Coefficient | SE | z | P > z | 95% CI |
|---|---|---|---|---|---|
| EQ5D score baseline | 0.5967924 | 0.0406532 | 14.68 | .000 | 0.5171136 to 0.6764713 |
| Patient gender | 0.0521357 | 0.0179587 | 2.90 | .004 | 0.0169372 to 0.0873341 |
| Age category, y | |||||
| 50-59 | −0.0187366 | 0.0338184 | −0.55 | .580 | −0.0850194 to 0.0475462 |
| 60-69 | −0.0039931 | 0.0318242 | −0.13 | .900 | −0.0663674 to 0.0583812 |
| 70-79 | −0.0108462 | 0.0331665 | −0.33 | .744 | −0.0758513 to 0.0541589 |
| > 80 | −0.0243156 | 0.0396575 | −0.61 | 0.540 | −0.1020428 to 0.0534116 |
| BMI category | |||||
| Overweight | −0.0100358 | 0.0166613 | −0.60 | 0.547 | −0.0426913 to 0.0226198 |
| Obese | −0.0069584 | 0.0182376 | −0.38 | 0.703 | −0.0427036 to 0.0287867 |
| Morbidly obese | −0.0652968 | 0.0651534 | −1.00 | 0.316 | −0.1929952 to 0.0624015 |
| Smoking status | |||||
| Previous | −0.0033888 | 0.0148166 | −0.23 | 0.819 | −0.0324288 to 0.0256512 |
| Current | −0.0069576 | 0.0239007 | −0.29 | 0.771 | −0.0538022 to 0.039887 |
| ECG result | −0.0057031 | 0.016846 | −0.34 | 0.735 | −0.0387207 to 0.0273145 |
| Celecoxib treatment | −0.0010674 | 0.0136832 | −0.08 | 0.938 | −0.0278859 to 0.0257511 |
| Diabetes | −0.0989409 | 0.0252237 | −3.92 | 0.000 | −0.1483784 to −0.0495034 |
| TCC history | −0.015477 | 0.0155777 | −0.99 | 0.320 | −0.0460086 to 0.0150547 |
| Year | |||||
| 2 | −0.0250881 | 0.0103193 | −2.43 | 0.015 | −0.0453134 to −0.0048627 |
| 3 | −0.0107493 | 0.0102873 | −1.04 | 0.296 | −0.0309119 to 0.0094134 |
| Tumor recurrence | |||||
| Unknown | 0.0334809 | 0.0823301 | 0.41 | 0.684 | −0.1278831 to 0.1948449 |
| Grade 1 | 0.062 0308 | 0.0555815 | 1.12 | 0.264 | −0.046907 to 0.1709685 |
| Grade 2 | 0.0518003 | 0.0339202 | 1.53 | 0.127 | −0.014682 to 0.1182826 |
| Grade 3 | −0.0830612 | 0.0258832 | −3.21 | 0.001 | −0.1337914 to −0.0323311 |
| Progression | −0.0990853 | 0.037488 | −2.64 | 0.008 | −0.1725605 to −0.0256102 |
| Progression history | 0.0043892 | 0.0516379 | 0.08 | 0.932 | −0.0968193 to 0.1055976 |
| Constant | 0.3218822 | 0.0489321 | 6.58 | 0.000 | 0.225977 to 0.4177873 |
Abbreviations: BMI = body mass index; CI = confidence interval; ECG = electrocardiography; EQ-5D = EuroQoL 5 dimension; HRQoL = health-related quality of life; SE = standard error; TCC = transitional cell carcinoma.
Supplemental Table 8.
Primary HRQoL Regression Including Time and Event Interaction
| EQ-5D Score | Coefficient | SE | z | P > z | 95% CI |
|---|---|---|---|---|---|
| EQ-5D score baseline | 0.5973907 | 0.0407683 | 14.65 | .000 | 0.5174862 to 0.6772952 |
| Patient gender | 0.0530437 | 0.0180207 | 2.94 | .003 | 0.0177238 to 0.0883636 |
| Age category, y | |||||
| 50-59 | −0.020057 | 0.0339243 | −0.59 | .554 | −0.0865473 to 0.0464333 |
| 60-69 | −0.0060109 | 0.03192 99 | −0.19 | .851 | −0.0685923 to 0.0565705 |
| 70-79 | −0.0113383 | 0.0332657 | −0.34 | .733 | −0.0765379 to 0.0538613 |
| >80 | −0.0274407 | 0.0397877 | −0.69 | .490 | −0.1054232 to 0.0505419 |
| BMI category | |||||
| Overweight | −0.01043 | 0.0167119 | −0.62 | .533 | −0.0431847 to 0.0223247 |
| Obese | −0.0073803 | 0.0183151 | −0.40 | .687 | −0.0432771 to 0.0285166 |
| Morbidly obese | −0.0629669 | 0.0653087 | −0.96 | .335 | −0.1909695 to 0.0650357 |
| Smoking status | |||||
| Previous | −0.0029297 | 0.0148631 | −0.20 | .844 | −0.0320609 to 0.0262014 |
| Current | −0.0080374 | 0.0239813 | −0.34 | .738 | −0.05504 to 0.0389651 |
| ECG result | −0.0052007 | 0.0168996 | −0.31 | .758 | −0.0383233 to 0.0279219 |
| Celecoxib treatment | −0.0004786 | 0.0137258 | −0.03 | .972 | −0.0273807 to 0.0264235 |
| Diabetes | −0.1000132 | 0.0253062 | −3.95 | .000 | −0.1496125 to −0.0504139 |
| TCC history | −0.0157322 | 0.0156268 | −1.01 | .314 | −0.0463602 to 0.0148957 |
| Tumor recurrence & year interactions | |||||
| No cancer & > year 1 | −0.0209844 | 0.0089722 | −2.34 | .019 | −0.0385696 to −0.0033992 |
| Unknown & year 1 | 0.0506889 | 0.1156377 | 0.44 | .661 | −0.1759568 to 0.2773346 |
| Unknown & > year 1 | 0.0038289 | 0.1159498 | 0.03 | .974 | −0.2234286 to 0.2310864 |
| Grade 1 & year 1 | −0.028671 | 0.0896046 | −0.32 | .749 | −0.2042929 to 0.1469509 |
| Grade 1 & > year 1 | 0.0907741 | 0.0706822 | 1.28 | .199 | −0.0477605 to 0.2293087 |
| Grade 2 & year 1 | 0.0284185 | 0.0468997 | 0.61 | .545 | −0.0635031 to 0.1203402 |
| Grade 2 & > year 1 | 0.0690765 | 0.0486986 | 1.42 | .156 | −0.026371 to 0.1645239 |
| Grade 3 & year 1 | −0.1096423 | 0.0317785 | −3.45 | .001 | −0.171927 to −0.0473577 |
| Grade 3 & > year 1 | −0.0429308 | 0.0428694 | −1.00 | .317 | −0.1269532 to 0.0410917 |
| Progression | −0.0937818 | 0.0373684 | −2.51 | .012 | −0.1670225 to −0.0205412 |
| Progression history | 0.0053212 | 0.051364 | 0.10 | .917 | −0.0953503 to 0.1059927 |
| Constant | 0.3228122 | 0.0490795 | 6.58 | .000 | 0.2266181 to 0.4190063 |
Abbreviations: BMI = body mass index; CI = confidence interval; ECG = electrocardiography; EQ-5D = EuroQoL 5-dimension; HRQoL = health-related quality of life; SE = standard error; TCC = transitional cell carcinoma.
Supplemental Table 9.
HRQoL Regression With Intermediate- and High-Risk Patients and Annual EQ-5D
| EQ-5D Score | Coefficient | SE | z | P > z | 95% CI |
|---|---|---|---|---|---|
| EQ-5D score baseline | 0.6222412 | 0.0440824 | 14.12 | .000 | 0.5358413 to 0.7086411 |
| Risk group | −0.0221777 | 0.0181653 | −1.22 | .222 | −0.0577811 to 0.0134256 |
| Patient gender | 0.0344006 | 0.0192668 | 1.79 | .074 | −0.0033617 to 0.0721629 |
| Age category, y | |||||
| 50-59 | −0.0421994 | 0.0414963 | −1.02 | .309 | −0.1235307 to 0.039132 |
| 60-69 | −0.051239 | 0.0393116 | −1.30 | .192 | −0.1282882 to 0.0258103 |
| 70-79 | −0.0600482 | 0.0411742 | −1.46 | .145 | −0.1407482 to 0.0206518 |
| >80 | −0.0726505 | 0.0482603 | −1.51 | .132 | −0.1672389 to 0.0219379 |
| BMI category | |||||
| Overweight | −0.0221557 | 0.018897 | −1.17 | .241 | −0.0591931 to 0.0148818 |
| Obese | −0.0381121 | 0.0206114 | −1.85 | .064 | −0.0785097 to 0.0022855 |
| Morbidly obese | −0.10648 | 0.0743021 | −1.43 | .152 | −0.2521095 to 0.0391495 |
| Smoking status | |||||
| Previous | 0.0102264 | 0.0171481 | 0.60 | .551 | −0.0233833 to 0.0438361 |
| Current | −0.0547593 | 0.0252457 | −2.17 | .030 | −0.1042399 to −0.0052786 |
| ECG result | −0.0382036 | 0.0187138 | −2.04 | .041 | −0.074882 to −0.0015251 |
| Celecoxib treatment | −0.0081224 | 0.0155085 | −0.52 | .600 | −0.0385186 to 0.0222737 |
| Diabetes | −0.0627696 | 0.027287 | −2.30 | .021 | −0.116251 to −0.0092881 |
| TCC history | −0.0177708 | 0.016869 | −1.05 | .292 | −0.0508335 to 0.0152919 |
| Year | |||||
| 2 | −0.0205743 | 0.009907 | −2.08 | .038 | −0.0399916 to −0.001157 |
| 3 | −0.0187082 | 0.0106461 | −1.76 | .079 | −0.0395742 to 0.0021579 |
| Tumor recurrence | |||||
| Unknown | 0.0616068 | 0.0957521 | 0.64 | .520 | −0.1260638 to 0.2492774 |
| Grade 1 | −0.005975 | 0.0317292 | −0.19 | .851 | −0.068163 to 0.056213 |
| Grade 2 | 0.0019765 | 0.0221878 | 0.09 | .929 | −0.0415108 to 0.0454638 |
| Grade 3 | −0.0434608 | 0.0277767 | −1.56 | .118 | −0.0979022 to 0.0109806 |
| Progression | −0.1020626 | 0.0428498 | −2.38 | .017 | −0.1860467 to −0.0180785 |
| Progression history | −0.0434159 | 0.0623099 | −0.70 | .486 | −0.1655411 to 0.0787093 |
| Constant | 0.4007673 | 0.0596421 | 6.72 | .000 | 0.2838709 to 0.5176637 |
Abbreviations: BMI = body mass index; CI = confidence interval; ECG = electrocardiography; EQ-5D = EuroQoL 5-dimension; HRQoL = health-related quality of life; SE = standard error; TCC = transitional cell carcinoma.
Supplemental Table 10.
Base Case HRQoL Regression Including Cystectomy as a Covariate
| EQ-5D Score | Coefficient | SE | z | P > z | 95% CI |
|---|---|---|---|---|---|
| EQ-5D score baseline | 0.636108 | 0.0377744 | 16.84 | .000 | 0.5620715 to 0.7101444 |
| Patient gender | 0.0477877 | 0.0163828 | 2.92 | .004 | 0.0156781 to 0.0798973 |
| Age category, y | |||||
| 50-59 | −0.0288782 | 0.0346037 | −0.83 | .404 | −0.0967001 to 0.0389438 |
| 60-69 | −0.037565 | 0.0327225 | −1.15 | .251 | −0.1016999 to 0.0265698 |
| 70-79 | −0.0464312 | 0.0342036 | −1.36 | .175 | −0.1134689 to 0.0206066 |
| >80 | −0.052182 | 0.0399786 | −1.31 | .192 | −0.1305387 to 0.0261747 |
| BMI category | |||||
| Overweight | −0.0177077 | 0.0156904 | −1.13 | .259 | −0.0484604 to 0.0130449 |
| Obese | −0.0150946 | 0.0172463 | −0.88 | .381 | −0.0488967 to 0.0187074 |
| Morbidly obese | −0.0273359 | 0.0608352 | −0.45 | .653 | −0.1465707 to 0.0918989 |
| Smoking status | |||||
| Previous | 0.0064122 | 0.0142 637 | 0.45 | .653 | −0.0215443 to 0.0343686 |
| Current | −0.0295427 | 0.0214073 | −1.38 | .168 | −0.0715001 to 0.0124148 |
| ECG result | −0.0215747 | 0.0157273 | −1.37 | .170 | −0.0523997 to 0.0092503 |
| Celecoxib treatment | −0.0081632 | 0.0129252 | −0.63 | .528 | −0.0334961 to 0.0171696 |
| Diabetes | −0.0881547 | 0.0233651 | −3.77 | .000 | −0.1339494 to −0.0423601 |
| TCC history | −0.022155 | 0.0137706 | −1.61 | .108 | −0.049145 to 0.0048349 |
| Year | |||||
| 2 | −0.0174281 | 0.0085872 | −2.03 | .042 | −0.0342587 to −0.0005975 |
| 3 | −0.0161801 | 0.0090078 | −1.80 | .072 | −0.033835 to 0.0014749 |
| Cystectomy | −0.1676828 | 0.0382576 | −4.38 | .000 | −0.2426664 to −0.0926992 |
| Constant | 0.3340947 | 0.0476971 | 7.00 | .000 | 0.24061 to 0.4275793 |
Abbreviations: BMI = body mass index; CI = confidence interval; ECG = electrocardiography; EQ-5D = EuroQoL 5-dimension; HRQoL = health-related quality of life; SE = standard error; TCC = transitional cell carcinoma.
Supplemental Table 11.
Costing Regression
| Total Costs | Coefficient | SE | z | P > z | 95% CI |
|---|---|---|---|---|---|
| Tumor recurrence | |||||
| Unknown | 1517.223 | 1729.041 | 0.88 | .380 | −1871.636 to 4906.082 |
| Grade 1 | 1217.438 | 415.9633 | 2.93 | .003 | 402.1653 to 2032.711 |
| Grade 2 | 1676.051 | 385.9831 | 4.34 | .000 | 919.5377 to 2432.564 |
| Grade 3 | 3956.667 | 829.3751 | 4.77 | .000 | 2331.122 to 5582.212 |
| Risk group, high risk | 1967.914 | 311.494 | 6.32 | .000 | 1357.397 to 2578.431 |
| Year | |||||
| 2 | −921.3536 | 251.7046 | −3.66 | .000 | −1414.686 to −428.0217 |
| 3 | −1514.189 | 233.9928 | −6.47 | .000 | −1972.806 to −1055.571 |
| Risk group & year | |||||
| High risk & year 2 | −1511.85 | 343.8087 | −4.40 | .000 | −2185.702 to −837.997 |
| High risk & year 3 | −1894.898 | 319.9745 | −5.92 | .000 | −2522.036 to −1267.759 |
| Progression | 5406.938 | 1400.335 | 3.86 | .000 | 2662.332 to 8151.544 |
| Progression history | 2269.138 | 806.8528 | 2.81 | .005 | 687.7356 to 3850.54 |
| TCC history | 91.53518 | 91.50393 | 1.00 | .317 | −87.80923 to 270.8796 |
| Patient gender | 162.3912 | 104.348 | 1.56 | .120 | −42.12716 to 366.9096 |
| Diabetes | −67.09895 | 147.0358 | −0.46 | .648 | −355.2838 to 221.0859 |
| Celecoxib treatment | −103.1504 | 90.55783 | −1.14 | .255 | −280.6405 to 74.33965 |
| Toxicity | |||||
| Mild condition | 190.4007 | 173.7812 | 1.10 | .273 | −150.2041 to 531.0055 |
| Moderate condition | 171.735 | 300.5923 | 0.57 | .568 | −417.415 to 760.885 |
| Celecoxib treatment & toxicity interaction | |||||
| Interaction | |||||
| 1 & Mild condition | 153.2397 | 242.295 | 0.63 | .527 | −321.6498 to 628.1292 |
| 1 & Moderate condition | 390.0575 | 387.7738 | 1.01 | .314 | −369.9651 to 1150.08 |
| Age, y | |||||
| 50-59 | 36.85634 | 193.8437 | 0.19 | .849 | −343.0704 to 416.7831 |
| 60-69 | 62.7073 | 177.3931 | 0.35 | .724 | −284.9767 to 410.3913 |
| 70-79 | −78.92821 | 182.5277 | −0.43 | .665 | −436.676 to 278.8195 |
| >80 | 59.02592 | 226.9982 | 0.26 | .795 | −385.8824 to 503.9342 |
| BMI | |||||
| Overweight | 207.6795 | 95.78029 | 2.17 | .030 | 19.95362 to 395.4054 |
| Obese | 258.0722 | 113.3226 | 2.28 | .023 | 35.96402 to 480.1804 |
| Morbidly obese | 1257.968 | 623.3053 | 2.02 | .044 | 36.31178 to 2479.624 |
| Smoking status | |||||
| Previous | −57.20011 | 97.19538 | −0.59 | .556 | −247.6996 to 133.2993 |
| Current | −241.9663 | 122.4042 | −1.98 | .048 | −481.8741 to −2.058529 |
| Constant | 2348.796 | 305.0676 | 7.70 | .000 | 1750.875 to 2946.718 |
Abbreviations: BMI = body mass index; CI = confidence interval; SE = standard error; TCC = transitional cell carcinoma.
Supplemental Table 12.
Variance–Covariance Matrix Base Case HRQoL Regression Analysis
| Variable | EQ-5D Base | Gender | Age, y |
BMI |
Smoking |
ECG Result |
Celecoxib |
Diabetes |
History |
Year |
Grade |
Progression | Progression History | Constant | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50-60 | 60-70 | 70-80 | >80 | Overweight | Obese | Morbidly Obese | Previous | Current | 2 | 3 | Unknown | 1 | 2 | 3 | ||||||||||
| EQ-5D baseline | 0.00165 | |||||||||||||||||||||||
| Gender | 0.0000 | 0.0003 | ||||||||||||||||||||||
| Age, y | ||||||||||||||||||||||||
| 50-60 | 0.0000 | −0.0001 | 0.0011 | |||||||||||||||||||||
| 60-70 | −0.0001 | −0.0001 | 0.0009 | 0.0010 | ||||||||||||||||||||
| 70-80 | 0.0000 | −0.0001 | 0.0009 | 0.0009 | 0.0011 | |||||||||||||||||||
| >80 | −0.0001 | −0.0001 | 0.0009 | 0.0009 | 0.0010 | 0.0016 | ||||||||||||||||||
| BMI | ||||||||||||||||||||||||
| Overweight | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | −0.0001 | 0.0003 | |||||||||||||||||
| Obese | 0.0001 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0003 | ||||||||||||||||
| Morbidly obese | −0.0001 | 0.0000 | −0.0001 | −0.0001 | 0.0000 | 0.0000 | 0.0002 | 0.0002 | 0.0042 | |||||||||||||||
| Smoking | ||||||||||||||||||||||||
| Previous | 0.0000 | 0.0000 | −0.0001 | −0.0001 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | −0.0001 | 0.0002 | ||||||||||||||
| Current | 0.0001 | −0.0001 | 0.0000 | 0.0000 | 0.0001 | 0.0000 | 0.0000 | 0.0000 | −0.0001 | 0.0001 | 0.0006 | |||||||||||||
| ECG result | 0.0000 | 0.0000 | 0.0000 | −0.0001 | −0.0001 | −0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0003 | ||||||||||||
| Celecoxib | 0.0000 | 0.0000 | 0.0000 | 0.0001 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | −0.0001 | 0.0000 | 0.0002 | |||||||||||
| Diabetes | 0.0001 | 0.0000 | 0.0000 | −0.0001 | −0.0001 | −0.0001 | 0.0000 | 0.0000 | −0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0006 | ||||||||||
| TCC history | 0.0001 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0001 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0002 | |||||||||
| Year | ||||||||||||||||||||||||
| 2 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0001 | ||||||||
| 3 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0001 | |||||||
| Grade | ||||||||||||||||||||||||
| Unknown | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0068 | ||||||
| 1 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0031 | |||||
| 2 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0012 | ||||
| 3 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | −0.0001 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0007 | |||
| Progression | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | −0.0001 | 0.0014 | ||
| Progression history | 0.0000 | 0.0000 | 0.0000 | 0.0000 | −0.0001 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0004 | 0.0027 | |
| Constant | −0.0014 | −0.0002 | −0.0008 | −0.0008 | −0.0008 | −0.0008 | −0.0001 | −0.0002 | 0.0000 | 0.0000 | −0.0001 | 0.0000 | −0.0001 | −0.0001 | −0.0001 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0024 |
Abbreviations: BMI = body mass index; CI = confidence interval; ECG = electrocardiography; EQ-5D = EuroQoL 5 dimension; HRQoL = health-related quality of life; SE = standard error; TCC = transitional cell carcinoma.
Supplemental Table 13.
Variance–Covariance Matrix Base Case Cost Regression Analysis
| Variable | Tumor Grade |
HR | Year |
#Year |
Progression | Progression History | TCC History | Gender | Diabetes | Celecoxib | Toxicity |
#Toxicity |
Age, y |
BMI |
Smoking |
Constant |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unknown | 1 | 2 | 3 | 2 | 3 | 2 | 3 | Mild | Moderate | Mild | Moderate | 50-60 | 60-70 | 70-80 | >80 | Overweight | Obese | Morbidly Obese | Previous | Current | |||||||||
| Tumor grade | |||||||||||||||||||||||||||||
| Unknown | 2989584 | ||||||||||||||||||||||||||||
| 1 | 3197.897 | 173025.4 | |||||||||||||||||||||||||||
| 2 | 455.0007 | 5153.004 | 148982.9 | ||||||||||||||||||||||||||
| 3 | 4751.888 | 1440.31 | 5766.639 | 687863.1 | |||||||||||||||||||||||||
| HR | −12,708.1 | 7947.394 | 6233.751 | −12,978.7 | 97,028.53 | ||||||||||||||||||||||||
| Year | |||||||||||||||||||||||||||||
| 2 | 128.5532 | 2513.226 | 5118.316 | 2360.683 | 45,236.91 | 63,355.2 | |||||||||||||||||||||||
| 3 | 417.7922 | 6185.773 | 9399.375 | 3398.295 | 46,386.92 | 47,142.29 | 54,752.65 | ||||||||||||||||||||||
| HR # year 2 | 12,903.19 | −2631.28 | −1898.03 | 13,670.3 | −92,607.2 | −61,490.2 | −44,871.9 | 118204.4 | |||||||||||||||||||||
| HR # year 3 | 10,099.37 | −5412.12 | −5798.32 | 12,242.9 | −94,413.4 | −44,807.9 | −51,859.6 | 91,808.03 | 102383.7 | ||||||||||||||||||||
| Progression | 3772.371 | 67.20135 | −12,774.8 | −87,906.4 | −11,840.8 | −1096.95 | −1184.22 | 6543.22 | 10,696.11 | 1,960,938 | |||||||||||||||||||
| Progression history | 3597.378 | 131.9645 | 1131.308 | 3651.328 | −756.922 | 429.5359 | 37.94244 | −3169.66 | −1512.31 | 47,298.56 | 651011.4 | ||||||||||||||||||
| TCC history | −3973.36 | 177.3514 | −249.955 | −618.622 | 1794.413 | 201.794 | 446.6992 | −80.3491 | −176.578 | −1414.4 | −2551.17 | 8372.969 | |||||||||||||||||
| Patient gender | −2929.97 | −77.8528 | 500.8079 | 550.0644 | −184.652 | 924.8961 | 1028.345 | −228.396 | −256.491 | −934.697 | −1450.44 | 634.9075 | 10,888.51 | ||||||||||||||||
| Diabetes | 174.1897 | 458.7002 | −786.564 | −1579.5 | 61.53177 | 93.05056 | −59.2129 | −127.267 | −313.961 | 1281.531 | −1474.4 | 518.0848 | 588.3711 | 21,619.53 | |||||||||||||||
| Celecoxib | 2048.935 | 402.4205 | −98.0007 | 920.2471 | 216.8488 | −36.8882 | −714.756 | 81.53749 | 647.6306 | 231.6324 | 515.0038 | −10.0378 | −759.794 | −1101.22 | 8200.721 | ||||||||||||||
| Toxicity | |||||||||||||||||||||||||||||
| Mild | −600.194 | 429.0679 | 321.6055 | −73.1877 | 630.1364 | 4588.025 | 5054.176 | −527.603 | −608.46 | 713.4595 | 1786.313 | 298.7808 | 2207.895 | −454.888 | 3779.813 | 30,199.89 | |||||||||||||
| Moderate | 2383.184 | −2263.53 | 1663.632 | 2924.52 | −1789.75 | 6931.381 | 6671.093 | 103.2672 | 3430.716 | 4155.016 | 1639.651 | 135.8265 | 1061.755 | −97.2649 | 3874.048 | 6976.896 | 90,355.71 | ||||||||||||
| Celecoxib # mild | 438.1648 | −1952.97 | 601.5322 | −1065.34 | −393.143 | −880.25 | 427.7197 | −287.85 | −203.163 | −1276.02 | −266.424 | 226.7488 | −1008.46 | 427.0523 | −7417.36 | −29,182.4 | −5234.27 | 58,706.88 | |||||||||||
| Celecoxib # moderate | −578.645 | 204.5142 | −1252.18 | −3234.89 | 3614.205 | −463.231 | 1200.66 | −1325.14 | −5724.77 | −4619.99 | −150.712 | −435.541 | −60.541 | 550.1123 | −7471.91 | −5457.64 | −88,242.4 | 9219.409 | 150368.5 | ||||||||||
| Age, y | |||||||||||||||||||||||||||||
| 50-60 | −461.326 | −1297.4 | −2167.35 | −219.887 | 877.5002 | −383.189 | −780.626 | −62.6462 | 536.8991 | 1446.35 | −632.307 | 54.15569 | −388.29 | −844.322 | 1911.414 | −315.633 | −16.8047 | −1357.87 | −284.003 | 37,575.4 | |||||||||
| 60-70 | −760.247 | −565.579 | −1189.19 | −1265.14 | 1569.057 | −532.382 | −836.337 | 80.84493 | 565.3626 | −535.485 | −3198.85 | 294.7149 | −1511.36 | −2402.45 | 2058.851 | −920.176 | −519.123 | −1076.13 | −61.9788 | 27,048.9 | 31,468.3 | ||||||||
| 70-80 | −3417.03 | −643.19 | −422.365 | −493.563 | 1144.489 | −680.322 | −1062.53 | 32.89036 | 515.9473 | −632.644 | −2133.45 | −233.831 | −1363.8 | −2793.31 | 1804.646 | −1484.46 | −1129.18 | 375.9521 | −995.23 | 26,940.94 | 27,342.93 | 33,316.37 | |||||||
| >80 | −2210.66 | −1113.08 | −169.061 | −1806.11 | 892.5153 | −297.992 | −451.897 | 16.11853 | 386.4642 | 1645.557 | −342.335 | 140.223 | −925.094 | −1278.83 | 2066.425 | −156.349 | 460.59 | −827.155 | −2429.76 | 27,010.24 | 27,247.6 | 27,388.06 | 51,528.18 | ||||||
| BMI | |||||||||||||||||||||||||||||
| Overweight | 3529.466 | 573.0519 | −339.4 | −552.401 | 250.3219 | 24.09107 | 739.0862 | 109.069 | −443.283 | 491.4164 | 290.0057 | −150.674 | −73.1737 | −792.842 | −161.863 | 724.8933 | −623.956 | −1356.18 | 1204.683 | −556.359 | −313.425 | 38.44737 | −1558.25 | 9173.863 | |||||
| Obese | 3521.786 | 400.006 | −595.534 | −726.146 | −612.32 | −32.3199 | 604.3832 | 90.40263 | −260.271 | −246.015 | 1816.794 | −373.677 | −373.834 | −3086.28 | −306.06 | 117.209 | −1073.1 | 28.07319 | 1280.047 | −2138.66 | −816.066 | 193.7971 | −609.137 | 5204.197 | 12,842.01 | ||||
| Morbidly obese | −118.211 | −9986.43 | −9469.14 | −2409.75 | −2017.09 | 40.76192 | 1044.331 | 456.362 | 986.1302 | 3133.209 | 1577.672 | 33.32034 | 4273.654 | −6030.72 | −2071.96 | 2339.3 | 19.31831 | −1655.31 | 1015.499 | 4159.043 | 3032.126 | 6170.16 | 5360.11 | 5745.526 | 6500.394 | 388509.5 | |||
| Smoking | |||||||||||||||||||||||||||||
| Previous | 3051.792 | −192.533 | −525.105 | 735.3619 | 147.8917 | −410.739 | −357.737 | 167.0641 | −65.6603 | −492.734 | 1603.186 | −316.844 | −1847.56 | −829.911 | 91.54407 | −1454.3 | −945.744 | 1053.433 | −61.0395 | −905.897 | −1423.65 | −677.65 | −1246.05 | −968.874 | -696.796 | −4306.3 | 9446.943 | ||
| Current | 3021.291 | 1286.492 | −351.976 | 619.1337 | 1659.942 | −449.125 | −294.024 | 231.7881 | −115.202 | −93.4865 | 785.4893 | 216.0982 | −2526.05 | −1053.26 | −581.953 | −978.082 | −1439.44 | 1179.949 | 787.8671 | 1593.945 | 1184.458 | 2831.625 | 2421.957 | 409.944 | 639.0463 | −575.662 | 5894.281 | 14,982.78 | |
| Constant | −650.554 | −8561.44 | −9019.26 | −3331.26 | −50,134.5 | −48,152.1 | −49,973.6 | 45,313.96 | 46,188.36 | 2439.925 | 2001.973 | −4320.44 | −6659.01 | 1944.855 | −5262.25 | −9175.75 | −10,217.5 | 5459.517 | 3663.643 | −26,755.5 | −26,623.3 | −26,969.7 | −26,842.4 | −4592.3 | −3397.78 | −9238.54 | −2227.81 | −6309.84 | 93,066.23 |
Abbreviations: BMI = body mass index; CI = confidence interval; ECG = electrocardiography; HR = high risk; HRQoL = health-related quality of life; SE = standard error; TCC = transitional cell carcinoma.
References
- 1.Ferlay J., Soerjomataram I., Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Research UK Bladder Cancer Statistics 2018. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bladder-cancer/incidence#heading-Three Available at: Accessed: March 27, 2018.
- 3.Leal J., Luengo-Fernandez R., Sullivan R., Witjes J.A. Economic burden of bladder cancer across the European Union. Eur Urol. 2016;69:438–447. doi: 10.1016/j.eururo.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 4.James A.C., Gore J.L. The costs of non-muscle invasive bladder cancer. Urol Clin North Am. 2013;40:261–269. doi: 10.1016/j.ucl.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Botteman M.F., Pashos C.L., Redaelli A., Laskin B., Hauser R. The health economics of bladder cancer. PharmacoEconomics. 2003;21:1315–1330. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 6.Svatek R.S., Hollenbeck B.K., Holmang S. The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol. 2014;66:253–262. doi: 10.1016/j.eururo.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Parkinson J.P., Konety B.R. Health related quality of life assessments for patients with bladder cancer. J Urol. 2004;172(Pt 1):2130–2136. doi: 10.1097/01.ju.0000139445.49500.24. [DOI] [PubMed] [Google Scholar]
- 8.Gerharz E.W., Mansson A., Mansson W. Quality of life in patients with bladder cancer. Urol Oncol. 2005;23:201–207. doi: 10.1016/j.urolonc.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Botteman M.F., Pashos C.L., Hauser R.S., Laskin B.L., Redaelli A. Quality of life aspects of bladder cancer: a review of the literature. Qual Life Res. 2003;12:675–688. doi: 10.1023/a:1025144617752. [DOI] [PubMed] [Google Scholar]
- 10.Wright J.L., Porter M.P. Quality-of-life assessment in patients with bladder cancer. Nat Clin Pract Urol. 2007;4:147–154. doi: 10.1038/ncpuro0750. [DOI] [PubMed] [Google Scholar]
- 11.Porter M.P., Penson D.F. Health related quality of life after radical cystectomy and urinary diversion for bladder cancer: a systematic review and critical analysis of the literature. J Urol. 2005;173:1318–1322. doi: 10.1097/01.ju.0000149080.82697.65. [DOI] [PubMed] [Google Scholar]
- 12.Kelly J.D., Tan W.S., Porta N. BOXIT—a randomised phase III placebo-controlled trial evaluating the addition of celecoxib to standard treatment of transitional cell carcinoma of the bladder (CRUK/07/004) Eur Urol. 2019;75:593–601. doi: 10.1016/j.eururo.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Oosterlinck W., Lobel B., Jakse G., Malmstrom P.U., Stockle M., Sternberg C. Guidelines on bladder cancer. Eur Urol. 2002;41:105–112. doi: 10.1016/s0302-2838(01)00026-4. [DOI] [PubMed] [Google Scholar]
- 14.Humphrey P.A., Moch H., Cubilla A.L., Ulbright T.M., Reuter V.E. The 2016 WHO classification of tumours of the urinary system and male genital organs—part B: prostate and bladder tumours. Eur Urol. 2016;70:106–119. doi: 10.1016/j.eururo.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 15.Lamm D.L., Blumenstein B.A., Crissman J.D. Maintenance bacillus Calmette-Guérin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group study. J Urol. 2000;163:1124–1129. [PubMed] [Google Scholar]
- 16.Tan W.S., Rodney S., Lamb B., Feneley M., Kelly J. Management of non-muscle invasive bladder cancer: a comprehensive analysis of guidelines from the United States, Europe and Asia. Cancer Treat Rev. 2016;47:22–31. doi: 10.1016/j.ctrv.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 17.EuroQol Group EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 18.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Dolan P., Gudex C., Kind P., Williams A. Centre for Health Economics, University of York; York, UK: 1995. A Social Tariff for EuroQol: Results From a UK General Population Survey. Working Papers. [Google Scholar]
- 20.Curtis L., Burns A. Personal Social Service Research Unit, University of Kent; Kent, UK: 2017. Unit Costs of Health and Social Care 2017. PSSRU Paper. [Google Scholar]
- 21.Manca A., Hawkins N., Sculpher M.J. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ. 2005;14:487–496. doi: 10.1002/hec.944. [DOI] [PubMed] [Google Scholar]
- 22.Briggs A.H., Parfrey P.S., Khan N. Analyzing health-related quality of life in the EVOLVE trial: the joint impact of treatment and clinical events. Med Decis Making. 2016;36:965–972. doi: 10.1177/0272989X16638312. [DOI] [PubMed] [Google Scholar]
- 23.Clarke P., Gray A., Legood R., Briggs A., Holman R. The impact of diabetes-related complications on healthcare costs: results from the United Kingdom Prospective Diabetes Study (UKPDS study no. 65) Diabet Med. 2003;20:442–450. doi: 10.1046/j.1464-5491.2003.00972.x. [DOI] [PubMed] [Google Scholar]
- 24.Drummond M.F., Sculpher M.J., Claxton K., Stoddart G.L., Torrance G.W. Oxford University Press; Oxford, UK: 2015. Methods for the Economic Evaluation of Health Care Programmes. [Google Scholar]
- 25.Wolowacz S.E., Briggs A., Belozeroff V. Estimating health-state utility for economic models in clinical studies: an ISPOR good research practices task force report. Value Health. 2016;19:704–719. doi: 10.1016/j.jval.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Liang K.-Y., Zeger S.L. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 27.Pan W. Akaike's information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 28.Cui J., Qian G. Selection of working correlation structure and best model in GEE analyses of longitudinal data. Commun Stat Simul Comput. 2007;36:987–996. [Google Scholar]
- 29.Boustead G.B., Fowler S., Swamy R., Kocklebergh R., Hounsome L., Section of Oncology, BAUS Stage, grade and pathological characteristics of bladder cancer in the UK: British Association of Urological Surgeons (BAUS) urological tumour registry. BJU Int. 2014;113:924–930. doi: 10.1111/bju.12468. [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni G.S., Finelli A., Fleshner N.E., Jewett M.A., Lopushinsky S.R., Alibhai S.M. Optimal management of high-risk T1G3 bladder cancer: a decision analysis. PLoS Med. 2007;4:e284. doi: 10.1371/journal.pmed.0040284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y., Denton B.T., Nielsen M.E. Comparison of surveillance strategies for low-risk bladder cancer patients. Med Decis Making. 2013;33:198–214. doi: 10.1177/0272989X12465353. [DOI] [PubMed] [Google Scholar]
- 32.Singer S., Ziegler C., Schwalenberg T., Hinz A., Gotze H., Schulte T. Quality of life in patients with muscle invasive and non-muscle invasive bladder cancer. Support Care Cancer. 2013;21:1383–1393. doi: 10.1007/s00520-012-1680-8. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt S., Frances A., Lorente Garin J.A. Quality of life in patients with non-muscle-invasive bladder cancer: one-year results of a multicentre prospective cohort study. Urol Oncol. 2015;33:19.e7–19.e15. doi: 10.1016/j.urolonc.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Yoshimura K., Utsunomiya N., Ichioka K., Matsui Y., Terai A., Arai Y. Impact of superficial bladder cancer and transurethral resection on general health-related quality of life: an SF-36 survey. Urology. 2005;65:290–294. doi: 10.1016/j.urology.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda T., Aptel I., Exbrayat C., Grosclaude P. Determinants of quality of life of bladder cancer survivors five years after treatment in France. Int J Urol. 2003;10:423–429. doi: 10.1046/j.1442-2042.2003.00657.x. [DOI] [PubMed] [Google Scholar]
- 36.Bohle A., Balck F., von Weitersheim J., Jocham D. The quality of life during intravesical bacillus Calmette-Guérin therapy. J Urol. 1996;155:1221–1226. [PubMed] [Google Scholar]
- 37.Sangar V.K., Ragavan N., Matanhelia S.S., Watson M.W., Blades R.A. The economic consequences of prostate and bladder cancer in the UK. BJU Int. 2005;95:59–63. doi: 10.1111/j.1464-410X.2005.05249.x. [DOI] [PubMed] [Google Scholar]
- 38.Cancer Research UK, Incisive Health Saving lives, averting costs: an analysis of the financial implications of achieving earlier diagnosis of colorectal, lung and ovarian cancer. 2014. https://www.cancerresearchuk.org/sites/default/files/saving_lives_averting_costs.pdf Available at: Accessed: March 27, 2018.
- 39.Yeung C., Dinh T., Lee J. The health economics of bladder cancer: an updated review of the published literature. Pharmacoeconomics. 2014;32:1093–1104. doi: 10.1007/s40273-014-0194-2. [DOI] [PubMed] [Google Scholar]
- 40.Stenzl A., Hennenlotter J., Schilling D. Can we still afford bladder cancer? Curr Opin Urol. 2008;18:488–492. doi: 10.1097/MOU.0b013e32830b8925. [DOI] [PubMed] [Google Scholar]
- 41.Chou R., Gore J.L., Buckley D. Urinary biomarkers for diagnosis of bladder cancer: a systematic review and meta-analysis. Ann Intern Med. 2015;163:922–931. doi: 10.7326/M15-0997. [DOI] [PubMed] [Google Scholar]
- 42.Tan W.S., Tan W.P., Tan M.Y. Novel urinary biomarkers for the detection of bladder cancer: a systematic review. Cancer Treat Rev. 2018;69:39–52. doi: 10.1016/j.ctrv.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Tan W.S., Teo C.H., Chan D. Mix methods approach to explore patients' perspectives on the acceptability of a urinary biomarker test in replacement of cystoscopy in bladder cancer surveillance. BJU Int. 2019;124:408–417. doi: 10.1111/bju.14690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mossanen M., Wang Y., Szymaniak J. Evaluating the cost of surveillance for non-muscle-invasive bladder cancer: an analysis based on risk categories. World J Urol. 2019;37:2059–2065. doi: 10.1007/s00345-018-2550-x. [DOI] [PubMed] [Google Scholar]
- 45.Babjuk M., Burger M., Compérat E. European Association of Urology Guidelines Office; Arnhem, The Netherlands: 2018. EAU guidelines on non-muscle-invasive bladder cancer (TaT1 and CIS) 2018. European Association of Urology Guidelines 2018 Edition. Presented at the EAU Annual Congress Copenhagen, 2018. [Google Scholar]
- 46.National Health Service National schedule of reference costs 2016-2017: NHS Improvement 2017. https://improvement.nhs.uk/resources/reference-costs/ Available at: Accessed: March 27, 2018.
- 47.Mowatt G., Zhu S., Kilonzo M. Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Health Technol Assess. 2010;14:1–331. doi: 10.3310/hta14040. iii-iv. [DOI] [PubMed] [Google Scholar]
- 48.National Institute for Health and Care Excellence (NICE) Lung cancer: the diagnosis and treatment of lung cancer (update). Implementing NICE guidance. 2011. Contract No. NICE clinical guideline 121. https://www.nice.org.uk/guidance/cg121 Available at: Accessed: March 27, 2018.