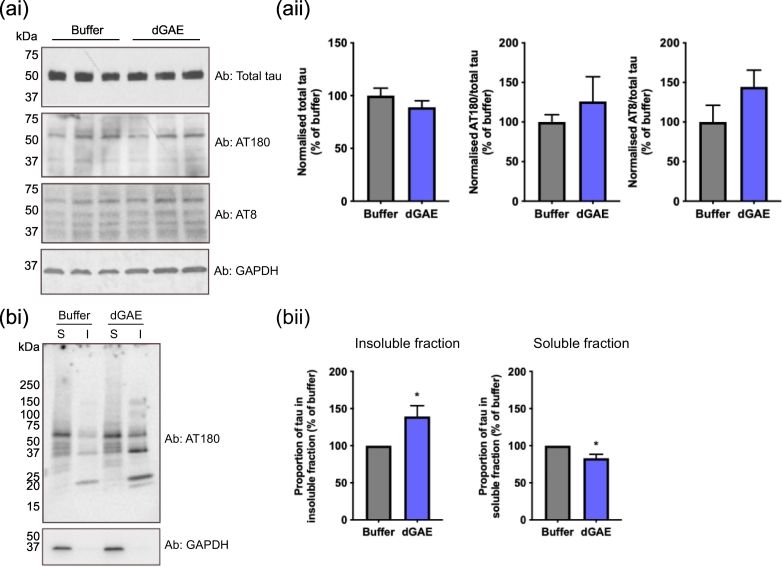
Figure 5.
Exposure to dGAE for 24 h leads to an accumulation of endogenous phospho-tau in the Triton-insoluble fraction of dSH-SY5Y cells. (ai) Representative Western blot of SH-SY5Y cell lysate. Soluble dGAE of 1 μM (unagitated) was added to the media of dSH-SY5Y cells. After 24 h, cells were lysed in RIPA buffer and run on SDS-PAGE. Blots were probed against total tau, AT180 and AT8. GAPDH was used as a loading control. (aii) The intensity of bands at 50–70 kDa were quantified for each antibody and normalised against GAPDH. The normalised values for AT180 and AT8 are expressed as a proportion of total tau (percentage of buffer). Data are shown as mean ± SEM from three independent experiments. An unpaired t-test shows no significant difference in total tau (p = .3101), AT180 (p = .4662) or AT8 (p = .2119) immunoreactivity between buffer-treated control and dGAE-treated cells. (bi) Cells were also sequentially lysed in Triton-X 100 lysis buffer and run on SDS-PAGE. S = Triton-X 100 soluble lysate; I = Triton-X 100 insoluble lysate. The AT180-antibody was used to detect endogenous phosphorylated tau and GAPDH was used as a loading control. (b) The proportion of tau in the insoluble and soluble fractions was quantified by densitometry and expressed as a percentage of buffer-treated controls. Data are shown as mean ± SEM from four independent experiments (one biological repeat per experiment). An unpaired t-test shows a significant difference in insoluble tau between buffer- (100 ± 0) and dGAE-treated cells (139.4 ± 14.54) (t = 2.709, df = 6, R2 = 0.5501, p = .0352) and in soluble tau between buffer (100 ± 0) and dGAE-treated cells (82.98 ± 5.48) (t = 3.106, df = 6, R2 = 0.6165, p = .0201).