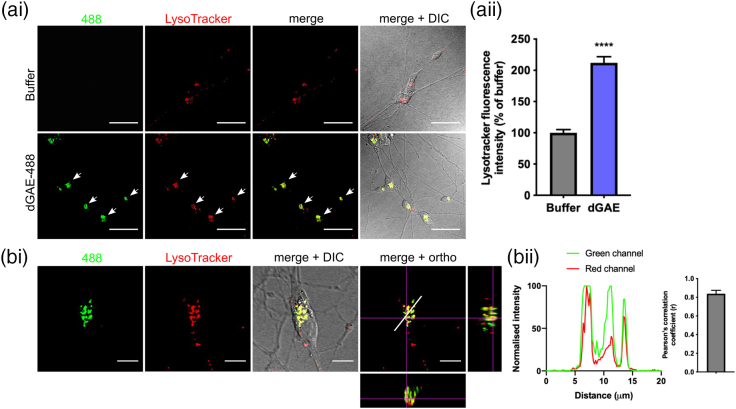
Figure 6.
Internalised soluble dGAE-488 is localised to acidic vesicles in dSH-SY5Y cells. (ai) Representative immunofluorescence images of cells exposed to 1 μM soluble dGAE-488 (unagitated) for 24 h. Cells were labelled with LysoTracker® to stain for acidic vesicles including lysosomes and endosomes, and were imaged live. dGAE-488 and LysoTracker® labelling is mainly localised in the cell body (white arrows). One z-slice is shown from the middle of the cell body. The scale bar represents 50 μm. (aii) Quantification of LysoTracker® fluorescence intensity as a percentage of buffer-treated cells. Data show mean ± SEM pooled from three independent experiments. N = 170 cells (buffer), N = 146 cells (dGAE). An unpaired t-test with Welch's correction shows a significant difference in LysoTracker® fluorescence intensity between dGAE (211.9% ± 9.89%) and buffer-treated cells (100% ± 5.23%) (t = 10, df = 222.6, R2 = 0.3101, p < .0001). (bi) Higher magnification of a single cell labelled with LysoTracker® containing internalised dGAE-488. The last panel displays orthogonal views to show colocalisation of dGAE-488 with LysoTracker®. One z-slice is shown from the middle of the cell body. The scale bar represents 10 μm. (bii) Normalised values of fluorescence intensity for 488 (green) and LysoTracker (red) along the region indicated by the white line (20 μm) in the last panel in (bi). Colocalisation of dGAE-488 and LysoTracker® is confirmed by the Pearson's correlation coefficient between the green and red channel. The average Pearson's R value was 0.8382 ± 0.036 (p < .0001 for each cell) (N = 7 cells).