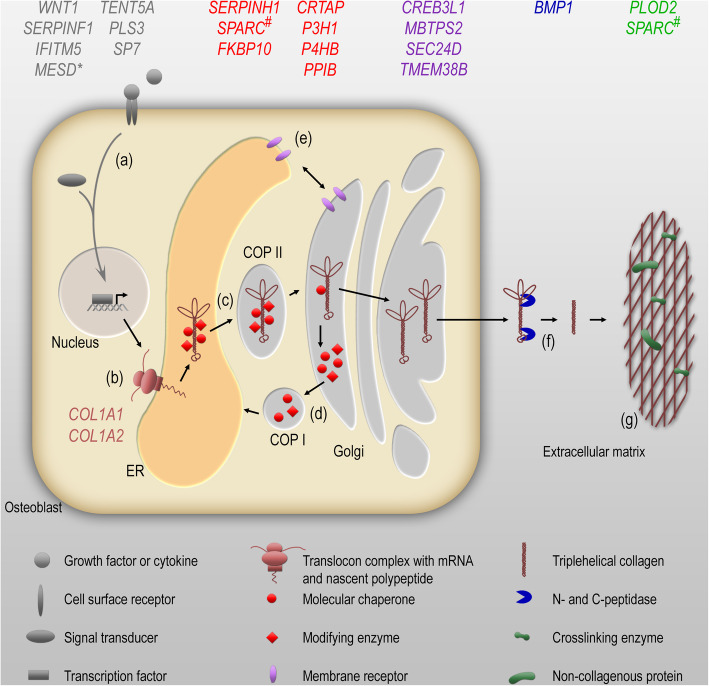
Fig. 1.
OI genes involved in collagen biosynthesis and maintenance of bone homeostasis. Growth factors and cytokine signal through cell surface receptors to initiate an intracellular signal cascade (a). The transduction of the signal results in the nuclear translocation of transcription factors to regulate the expression of genes involved in osteoblast differentiation or function and collagen biosynthesis. Several regulated genes encode proteins that upon secretion influence osteoclast formation and activity (RANKL, osteoprotegerin, sclerostin; not shown). The α1- and α2-chains of collagen type I, the main collagen produced in osteoblasts, are translated into the rough endoplasmic reticulum (ER) (b). Molecular chaperones support the folding of collagen chains enabling hydroxylation of proline and lysine residues by hydroxylases as well as subsequent glycosylation that is indispensable for proper formation of triplehelical collagen (c). The procollagen is secreted by a coat protein complex II (COP II)-mediated vesicular transport through the Golgi network into the extracellular space. Molecular chaperones and modifying enzymes dissociate pH-dependently from procollagen in the Golgi intermediate compartment and circulate COP I-mediated back to the ER (d). Integral proteins in the ER and Golgi membranes, such as ion channels or ER stress sensors, maintain the intracellular homeostasis and in that way preserve the secretory pathway (e). The N- and C-propeptides of the secreted procollagen are cleaved off by extracellular peptidases (f) and the processed, mature collagen molecules form a collagen network that is crosslinked and mineralized. Several collagenous and non-collagenous proteins interact with type I collagen to form the bone extracellular matrix (ECM) (g). Genes, in which mutations have been linked to osteogenesis imperfecta or related diseases, are indicated. These genes are involved in signal transduction and gene expression (grey), translation (brown), post-translational modification (red), ER homeostasis (purple), proteolytic processing (blue) or ECM structure, and mineralization (green). *MESD is an ER-resident chaperone for LRP proteins, co-receptors of the WNT signaling pathway. #SPARC can act as a chaperone in the ER as well as support mineralization of the collagen matrix extracellularly