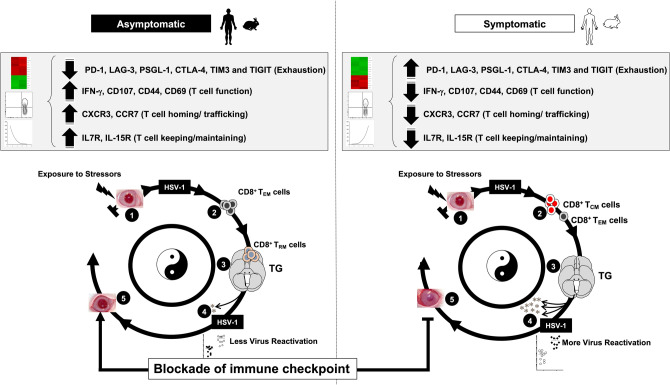
Figure 7.
TG-resident HSV-specific memory CD8+ TRM cells downregulate the T cell exhaustion associated pathway and confer protection from ocular herpes in HSV-1 infected asymptomatic humans and HLA transgenic rabbits. (1) Upon exposure to stressors, the HSV-1 enters into the cornea and travels through neurons to Trigeminal ganglia. (2) Following primary HSV-1 infection, the vast majority (up to 95%) of antiviral effector CD8+ T cells die, leaving behind only about 5% of CD8+ T cells destined to differentiate into a heterogeneous pool of memory CD8+ T cells. (3) The effector memory (TEM) and tissue-resident memory (TRM) CD8+ T-cell subsets are found mainly in the HSV-infected but "naturally protected" asymptomatic subjects, whereas the lymphoid organ-resident central memory (TCM) CD8+ T cell subsets are mainly present in non-protected Symptomatic subjects. (4) Reduced viral reactivation was observed among asymptomatic subjects possessing a higher frequency of CD8+ TRM cells resulting in a less severe herpes disease. (5) The findings study suggests that by blocking immune checkpoints, there is a reduced expression of T cell exhaustion molecules (PD-1, LAG-3, PSGL-1, CTLA-4, TIM3, and TIGIT) and T cell exhaustion associated Cell Adhesion Molecule pathway and increased retention of CD8+ TRM cell population in asymptomatic subjects. This memory CD8+ T cell population mediates recall responses and halts attempts of virus reactivation in the infected TG, thus accelerating viral clearance. More-so, reduced expression of T cell exhaustion pathway also gives rise to higher expression of genes associated with T cell function (CD107, IFN-γ), T cell homing (CXCR3, CCR7), and T-cell keeping (IL7R, IL15R). This helps in reducing the ocular herpes infection and recurrent herpetic disease.