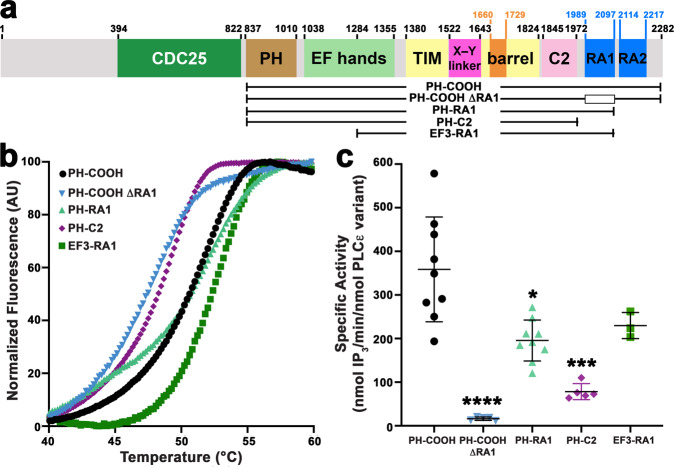
Fig. 1. The PLCε RA domains have different roles in stability and basal activity.
a Domain structure of rat PLCε. The residue numbers above the diagram correspond to predicted (CDC25-EF1/2) or observed (6PMP, this study) domain boundaries. PLCε variants used in this study are diagrammed below. The open box in PLCε PH-COOH ΔRA1 corresponds to deletion of the RA1 domain. b Representative thermal denaturation curves of PH-COOH (black circles), PH-COOH ΔRA1 (blue inverted triangles), PH-RA1 (light green triangles), PH-C2 (purple diamonds), and EF3-RA1 (dark green squares). The most dramatic decreases in thermal stability are in variants that lack the RA1 domain. c Loss of both RA domains (PH-C2) or the RA1 domain (PH-COOH ΔRA1) decreases basal-specific activity up to ∼20-fold relative to PH-COOH, whereas deletion of RA2 (PH-RA1) only decreased activity ∼2-fold. Each data point shown represents the average of the duplicates from one technical repeat. Error bars reflect SD. Data for PLCε PH-COOH was previously reported22. Significance was determined using a one-way ANOVA followed by Dunnett’s multiple comparisons test vs. PLCε PH-COOH. (****p ≤ 0.0001, ***p ≤ 0.0005, **p ≤ 0.001, *p ≤ 0.05).