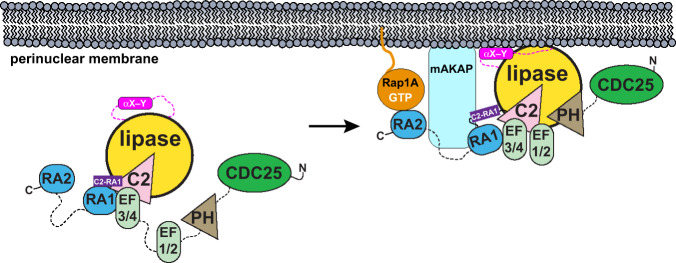
Fig. 4. Proposed model of basal PLCε regulation at the perinuclear membrane.
(Left) PLCε is present predominantly in the cytoplasm, and is maintained in a low-activity state by the autoinhibitory X–Y linker (hot pink) and the C2-RA1 linker (purple). The CDC25, PH, and RA2 domains, along with EF1/2, are not essential for full basal activity and may be flexibly connected to, or only transiently interact with, the PLCε core18,22,32,33. (Right) Localization of PLCε to the perinuclear membrane through interactions between the RA1 domain and the scaffolding protein mAKAP increase lipase activity17,19. RA1 binding to mAKAP could alter the conformation of, or displace, the C2-RA1 linker, increasing basal activity. Membrane association would also increase basal activity via interfacial activation12, which may be facilitated by interactions between the αX–Y helix and the membrane or, alternatively, with other domains in PLCε or proteins at the target membrane, such as activated Rap1A.