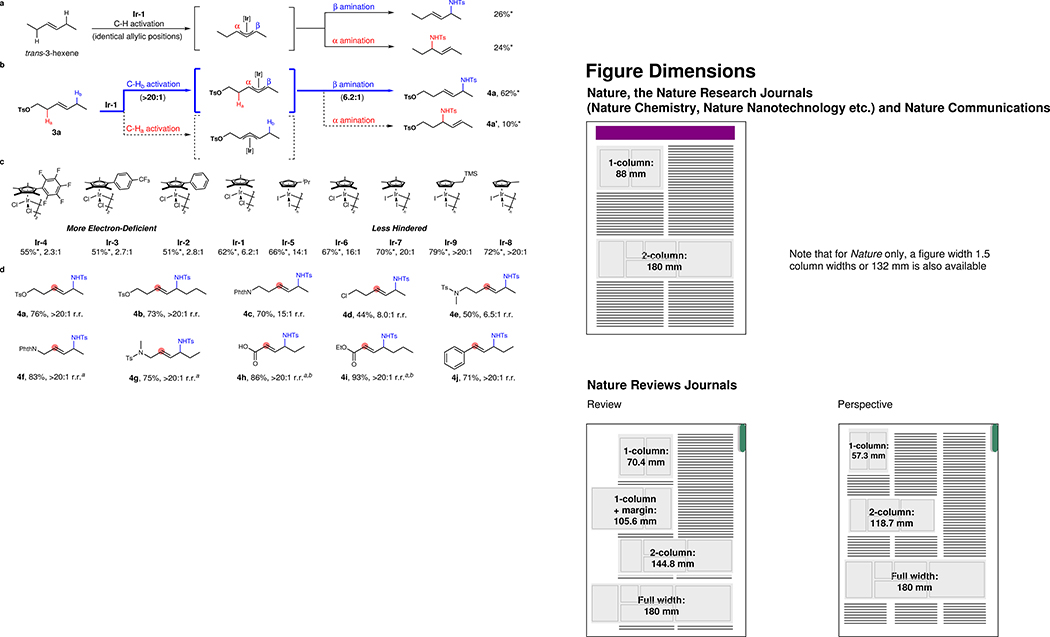
Figure 2. Study of regioselectivities in C–H amination of unsymmetrical trans-1,2-disubstituted alkenes.
a, Non-selective C–H amination of trans-3-hexene. b, Remote electronically controlled C–H activation and amination. c, Electronic and steric effects of Cp ligands with substrate 3a. d, Scope of unsymmetrical trans-1,2-disubstituted alkenes catalyzed by Ir-9. Unless otherwise noted, all reactions were conducted on a 0.1 mmol scale using alkene (1.0 equiv.), TsN3 (1.5 equiv.), [Ir-n] (15 mol% of monomer), AgBF4 (60 mol%), LiOAc (1.0 equiv.). Yields were determined after column chromatography. Yields* and r.r. values (β/α) were collected from 1H NMR spectrum of unpurified reaction mixutre with mesitylene as the internal standard. a5 mol% [Cp™IrCl2]2 (Ir-6) was used. bReaction was conducted at 50 °C.