Coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 can result in severe disease and become critically challenging to hospitals via high demand for intensive care and mechanical ventilation.
Guillain-Barré syndrome (GBS) and its variants have been described as neurologic complications of COVID-19, and fatal cases were reported.1 The mechanisms by which COVID-19 predisposes to autoimmunity are unclear, and potential biomarkers or risk factors remain unknown.
Case report
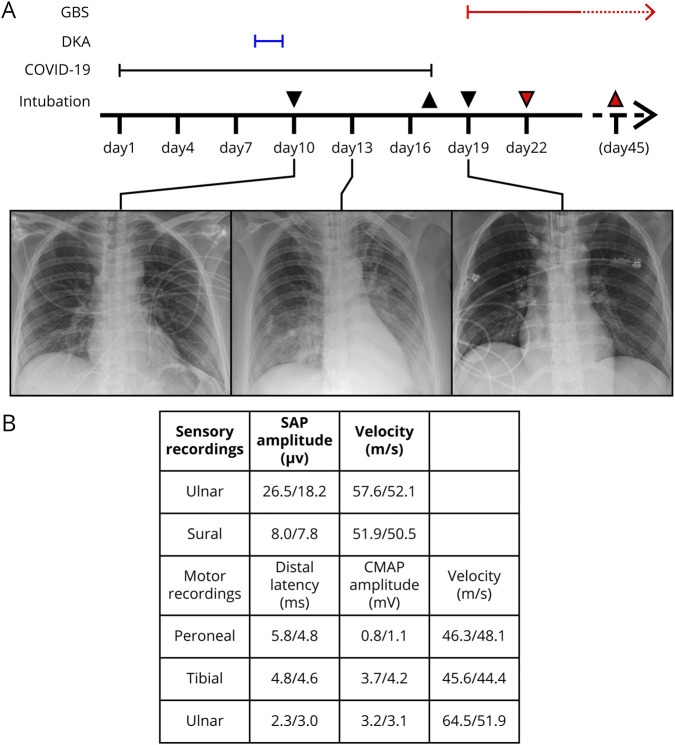
A 35-year-old, healthy Caucasian woman initially presented with fever and coughing over 1 week (days 0–7, figure, A for timeline) at her family doctor and was subsequently tested positive for SARS-CoV-2 via PCR. The patient had experienced no other infections in previous weeks and had not received any vaccinations.
Figure. Clinical course of our patient with COVID-19.
(A) Clinical course of our patient. Downward arrows indicate intubation, upward arrow indicates extubation, colored arrows indicate tracheostomy and decannulation. Chest x-ray images refer to the respective dates. (B) Data from nerve conduction studies on day 20. *Abnormal. Reference for CMAP is >5 mV in all examined nerves. EMG was not performed. COVID-19 = coronavirus disease 2019; CMAP = compound muscle action potential; DKA = diabetic ketoacidosis; GBS = Guillain-Barré syndrome; SAP = sensory action potential.
On day 8, she developed severe diabetic ketoacidosis (DKA) as the first manifestation of type 1 diabetes (T1D; pH: 6.7; base excess: −27 mmol/L; blood glucose: 25.1 mmol/L, HbA1c: 6.4%), and antibodies against islet cell antigen 2 and glutamate decarboxylase (GAD65) were positive. There was no history of polyuria or polydipsia. She was admitted to intensive care unit and transiently recovered after fluid resuscitation and insulin treatment.
However, because of rapidly developing respiratory insufficiency, intubation became necessary on day 10. Chest x-ray and CT scan showed signs suggestive of COVID-19.
During ICU treatment, the patient recovered from COVID-19, and nasopharyngeal swabs for SARS-CoV-2 were repeatedly negative (first on day 15). The patient was weaned from ventilatory support, and extubation was performed on day 17. Blood glucose levels remained well controlled throughout after IV insulin substitution.
However, restrictive lung disorder resulting from diaphragmatic paralysis rapidly evolved, and poor management of pharyngeal secretions forced reintubation within 24 hours. A rapidly progressive proximal tetraparesis and dysautonomia with tachycardia and absent heart rate variability were first noticed on day 19 and repeatedly let to self-limiting, symptomatic bradycardia on day 20.
MRI scans of the brain and cervical cord were unremarkable. CSF analysis showed albuminocytologic dissociation (CSF total protein: 1421 mg/L, CSF cell count: 2/µL, and CSF oligoclonal bands were negative). SARS-CoV-2 was not detectable in the CSF. Nerve conduction studies revealed widespread axonal damage as indicated by reduced compound motor action potentials (figure, B). Antibodies against Gd1B were positive, whereas antibodies against other gangliosides were negative.
Laboratory analysis revealed selective immunoglobulin A deficiency (sIgAD; serum IgA <0.05g/L) but was otherwise unremarkable for autoimmune or hematologic disorders. Virologic and serologic testing was negative for hepatitis, HIV, herpes viruses, other respiratory viruses, and Campylobacter.
We performed 5 courses of plasma exchange. After this, we observed early recovery with increasing muscle strength and reemerging muscle reflexes. However, bulbar palsy persisted and prompted early tracheostomy on day 22. Decannulation was performed on day 45. Currently, symptoms mostly resolved but mild neurogenic dysphagia persists. C-peptide levels remain below thresholds, and insulin treatment is continued.
Discussion
We believe that our patient suffered from sIgAD since childhood but remained undiagnosed in the absence of symptoms until autoimmunity was finally induced by COVID-19.
SARS-CoV-2 has been identified as a potential trigger for GBS because its spike viral protein interacts with ganglioside antigens on human cells and thereby paves the way for an antiganglioside immune reaction.1 Such association has not been shown yet for T1D, but previous reports on the development of DKA within already 1 week after nivolumab treatment2 indicate that a single trigger can result in rapid-onset T1D in susceptible patients and normal HbA1c levels render preexisting yet undiscovered T1D unlikely here. In addition, sIgAD is a known risk factor for T1D.3
Moreover, previous cases on GBS after COVID-19 mostly showed an onset within 5–10 days supporting that COVID-19 is a sufficient trigger of rapid-onset autoimmunity.1 However in GBS, the role of sIgAD is less clear, although asymptomatic ganglioside antibodies are more common in patients with sIgAD compared with the general population.4
Clinically, our case here shares many features with previously published reports. Our patients suffered from a predominantly axonal variant of GBS, which has been observed before. Previous patients also developed GBS already within 7–10 days after the onset of COVID-19.1 Furthermore, bulbar involvement has been described in previous cases.5,6 Especially, it was present in a case of Miller-Fisher syndrome that also presented with Gd1b antibodies.5 We have not observed ophthalmoplegia, yet dysphagia subsequent to bulbar palsy resulted in necessity for tracheostomy in our patient as described before.7
Finally, our findings expand the spectrum of autoimmunity after COVID-19. Although COVID-19 was repeatedly demonstrated as an inducer of GBS alone, we believe that the preexisting sIgAD significantly contributed to the development of 2 different autoimmune disorders after COVID-19.
Appendix. Authors
Footnotes
COVID-19 Resources: NPub.org/COVID19
Study funding
No targeted funding reported.
Disclosure
S. Pfeuffer received travel reimbursements from Sanofi Genzyme and Merck Serono; honoraria for lecturing from Sanofi Genzyme, Biogen, and Mylan Healthcare; and research support from Merck Serono, Diamed, and the German Multiple Sclerosis Society. M. Pawlowski and G.S. Joos declare no conflicts of interest. J. Minnerup received grants from EVER Pharma Jena GmbH and Ferrer International; travel grants from Boehringer Ingelheim; and speaking fees from Bayer Vital and Chugai Pharma. S.G. Meuth received honoraria for lecturing and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer Health Care, Biogen, Celgene, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi Aventis, Chugai Pharma, QuintilesIMS, and Teva. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, German Academic Exchange Service, Hertie Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, German Foundation Neurology and Almirall, Amicus Therapeutics Germany, Biogen, Diamed, Fresenius Medical Care, Genzyme, Merck Serono, Novartis, ONO Pharma, Roche, and Teva. R. Dziewas declares no conflicts of interest. H. Wiendl received compensation for serving on Scientific Advisory Boards/Steering Committees for Bayer Healthcare, Biogen Idec, Sanofi Genzyme, Merck Serono, and Novartis; he has received speaker honoraria and travel support from Bayer Vital GmbH, Bayer Schering AG, Biogen, CSL Behring, EMD Serono, Fresenius Medical Care, Genzyme, Merck Serono, Omniamed, Novartis, and Sanofi Aventis. He has received compensation as a consultant from Biogen Idec, Merck Serono, Novartis, Roche, and Sanofi Genzyme. Heinz Wiendl also received research support from Bayer Healthcare, Bayer Vital, Biogen Idec, Merck Serono, Novartis, Sanofi Genzyme, Sanofi US, and Teva. Go to Neurology.org/NN for full disclosures.
References
- 1.Dalakas MC. Guillain-Barre syndrome: the first documented COVID-19-triggered autoimmune neurologic disease: more to come with myositis in the offing. Neurol Neuroimmunol Neuroinflamm 2020;7:e781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee S, Morgan A, Shah S, Ebeling PR. Rapid-onset diabetic ketoacidosis secondary to nivolumab therapy. Endocrinol Diabetes Metab Case Rep 2018;2018:18-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang N, Shen N, Vyse TJ, et al. . Selective IgA deficiency in autoimmune diseases. Mol Med 2011;17:1383–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barka N, Shen GQ, Shoenfeld Y, et al. . Multireactive pattern of serum autoantibodies in asymptomatic individuals with immunoglobulin A deficiency. Clin Diagn Lab Immunol 1995;2:469–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costello F, Dalakas MC. Cranial neuropathies and COVID-19: neurotropism and autoimmunity. Neurology Epub 2020 Jun 2. [DOI] [PubMed]
- 6.Gutierrez-Ortiz C, Mendez A, Rodrigo-Rey S, et al. . Miller Fisher Syndrome and polyneuritis cranialis in COVID-19. Neurology Epub 2020 Apr 17. [DOI] [PubMed]
- 7.Schroder JB, Marian T, Muhle P, et al. . Intubation, tracheostomy, and decannulation in patients with Guillain-Barre-syndrome-does dysphagia matter? Muscle Nerve 2019;59:194–200. [DOI] [PubMed] [Google Scholar]