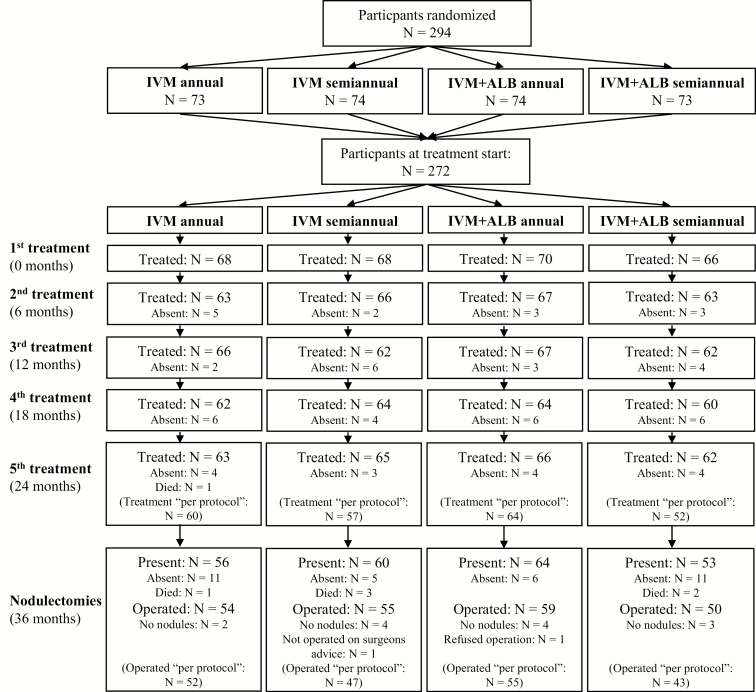
Figure 2.
Participant flow chart, showing the number of participants randomized, treated, and operated. Participants who were absent for 1 or more treatments were always invited to continue treatment at the next visit or to come for the nodulectomies. Therefore, the number of absent participants changed between the respective visits. All participants that took part in the treatment or nodulectomies with no major violations to the protocol are listed in parentheses as “per protocol.” In total, 294 patients were randomized, but 22 participants did not take part in the treatment at all, due to pregnancy (n = 1), traveling (n = 10), refusal to participate (n = 3), moving (n = 3), and medical reasons (n = 5). To reach the initially planned number of 272 participants, 22 additional patients were consecutively randomly assigned. The first treatment (n = 272) was carried out in 2 batches: the first group (n = 182) was treated from 9–22 February 2013 and the second group (n = 90) from 6–13 April 2013. The second treatment (n = 259; 95.2%) was carried out 5.5 ± 0.6 months (range 5–8) after the first treatment. The third treatment (n = 257; 94.5%) was carried out 12 ± 0.8 months (range 11–13) after the first treatment and 5.9 ± 0.9 months (range 4–7) after the second treatment. The fourth treatment (n = 251; 92.3%) was carried out 17.8 ± 0.8 months (range 16–19) after the first treatment and 5.4 ± 0.5 months (range 4–6) after the third treatment. The fifth treatment (n = 254; 93.4%) was carried out 23.8 ± 0.8 months (range 22–25) after the first treatment and 5.7 ± 0.5 months (range 5–6) after the fourth treatment. The nodulectomies (n = 233; 85.7%) were carried 35.4 ± 0.9 months (range 34–36) after the first treatment and 10.9 ± 0.4 months (range 10–12) after the fifth treatment. Abbreviations: ALB, albendazole; IVM, ivermectin.