Abstract
Background
Several countries have implemented a 2-dose (2D) human papillomavirus (HPV) vaccination schedule for adolescents based on immunobridging studies. We compared immunogenicity of 2D vs 3-dose (3D) schedules of the quadrivalent vaccine (4vHPV) up to 10 years after the first dose.
Methods
Girls aged 9–13 years were randomized to receive 2D or 3D and were compared with women aged 16–26 receiving 3D at day 1 and months 7, 24, and 120 after the first dose. Antibody levels for HPV6/11/16/18 were evaluated using the competitive Luminex immunoassay (cLIA) and total immunoglobulin G assay. Geometric mean titers (GMTs) and seropositivity rates were compared between the different groups at different time points. Noninferiority of GMT ratios was defined as the lower bound of the 2-sided 95% confidence interval (CI) being greater than 0.5. Kinetics of antibody titers over time among study groups were examined.
Results
At 120 months, data from 35 2D girls, 38 3D girls, and 30 3D women were used for analyses. cLIA seropositivity rates were above 95% for all HPV vaccine types and all schedules, except HPV18, with the lowest seropositivity observed among 3D women (60.0%; 95% CI, 40.6%–77.3%). GMT ratios (cLIA) for both 2D and 3D girls were noninferior to 3 doses in women for HPV6/11/16/18. Trends were comparable between assays.
Conclusions
GMTs for HPV6/11/16/18 after 2D or 3D of 4vHPV in girls were noninferior to 3D in adult women up to 120 months postvaccination. This study demonstrates long-term immunogenicity of the 2D HPV vaccine schedule.
Keywords: immunogenicity, papillomavirus infections, human papillomavirus vaccine, immunization schedule
Up to 120 months post-vaccination antibody titers in girls who received 2 or 3 doses of human papillomavirus vaccine were noninferior to those in young women who received 3 doses, the group in which clinical efficacy has been shown.
Human papillomavirus (HPV) is the most common sexually transmitted infection, with a lifetime risk of infection of approximately 80% [1]. HPV infections are the cause of genital warts and cervical cancer and are strongly associated with anal, penile, oropharyngeal, vaginal, and vulvar cancers [2]. Currently, 3 prophylactic HPV vaccines are available: bivalent, quadrivalent, and nonvalent. All 3 vaccines were originally approved in 3-dose (3D) schedules (0, 1 or 2, and 6 months). Given the lack of a correlate of protection and the long time between onset of an HPV infection and most of its sequelae, the International Agency for Research on Cancer has endorsed several intermediate endpoints for the evaluation of HPV vaccines. To assess the effect of the HPV vaccine in women aged 16–26 years, originally cervical intraepithelial neoplasia grade 2 (CIN2+) or higher was used as an outcome. Now, persistent infections for 6 months or longer are also considered as an outcome for individuals aged ≥16 years. For evaluation in individuals aged <16 years, an immunobridging principle is used. Immunobridging assumes that if the immunogenicity can be shown to be comparable between groups, the efficacy will be comparable also [3]. For the HPV vaccine, this means that if antibody responses in young adolescents aged 9–15 years are comparable to the antibody responses in young adults aged ≥16 years, among whom clinical efficacy against CIN2+ has been shown, effectiveness is assumed to be comparable [4, 5]. Hence, based on immunobridging studies, 2 doses of vaccine have been recommended for girls aged <15 years [6, 7].
Recommendation for the 2-dose (2D) schedule was based on noninferior immunogenicity among girls compared with 3 doses for women up to 26 years, as described above [7–9]. Subsequently, many countries have implemented the 2-dose schedule in their immunization programs [10]. However, continued monitoring of the duration of antibody persistence has been advised [11, 12]. Follow-up of the 2D schedule for 60 months after vaccination has been reported. It was found that there was no significant difference in the decline in antibody titers from 36 to 60 months in girls who received 2 or 3 doses, indicating a comparable decay in antibody levels [11]. However, longer-term antibody duration has not been reported for 2 doses. Here, we report the antibody responses up to 120 months after vaccination with 2D or 3D of the quadrivalent HPV (4vHPV) vaccine.
METHODS
Study Participants
This was a post hoc analysis of a phase 3, postlicensure, age-stratified, noninferiority immunogenicity trial. The study included 3 groups that received the 4vHPV vaccine: girls aged 9–13 years who were randomized to receive either 2 doses or 3 doses (2D and 3D girls) and women aged 16–26 years who received 3 doses (3D women) [7, 11]. At 60 months postvaccination, only the 2D and 3D girls were compared. Participants were eligible for this 120-month follow-up study if they agreed to be contacted for future studies at the start of the original trial and if they also contributed to the 60-month evaluation for 2D and 3D girls [11]. At 120 months postvaccination, eligible participants received an invitation letter and a follow-up phone call. Consenting participants provided a blood sample for serology. For the analyses reported here, data from participants were only included if they contributed to the study visits (day 1, months 7, 24, and 120) where data from all participants were collected. The relevant ethics boards of British Columbia, Nova Scotia, and Quebec approved the follow-up study.
Laboratory Analysis
Blinded antibody data for all time points were provided by Merck & Co, Inc, Kenilworth, New Jersey, at no cost for the study. Antibodies against HPV6, 11, 16, and 18 for all participants were measured using both the competitive Luminex immunoassay (cLIA) and total immunoglobulin G (IgG) assay (TIgG) [13]. Seropositivity rates and geometric mean titers (GMTs) were calculated among participants at 120 months; seropositivity rates and GMTs for earlier time points postvaccination have been previously reported [7, 11]. Seropositivity cutoffs for the cLIA were 20 mMU/mL, 16 mMU/mL, 20 mMU/mL, and 24 mMU/mL for HPV6, 11, 16, and 18, respectively [14]. Seropositivity cutoffs for the TIgG were 15 mMU/mL, 15 mMU/mL, 7 mMU/mL, and 10 mMU/mL for HPV6, 11, 16, and 18, respectively [15].
Statistical Analyses
Seropositivity rates and GMTs were compared between the different study groups for each HPV vaccine type at day 1 and months 7, 24, and 120 postvaccination. For GMT ratios, noninferiority was defined as the lower bound of the 2-sided 95% confidence interval (CI) around the GMT ratio being greater than 0.5, when comparing the GMTs for 2D and 3D girls with those in women. [16–18]. The paired sample t test and the 2-sample t test with unequal variances were used to explore the difference over time and to compute the P values based on the log scale of GMT values. Linear mixed-effects models on the assay natural log-transformed values were used to explore a possible difference in antibody decay over time between the groups. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc).
RESULTS
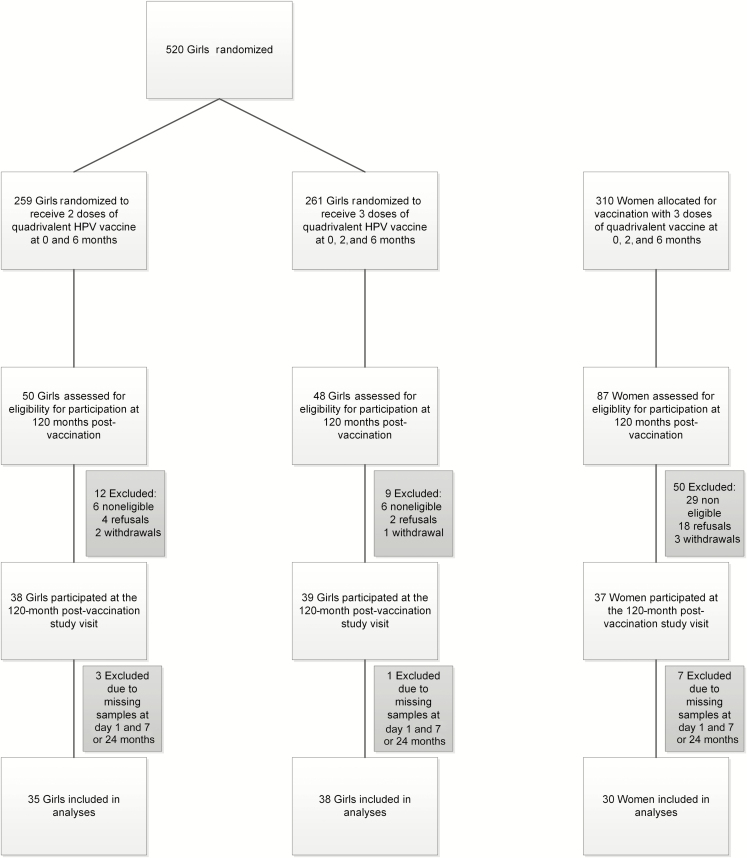
The flow of original study participants and of those included in the follow-up is shown in Figure 1. A total of 210 participants were approached for the 120-month follow-up visit, and 114 (54.2%) agreed to participate (2D girls, n = 38; 3D girls, n = 39; 3D women, n = 37). After exclusion of those who did not participate at all study visits (ie, day 1 and months 7, 24, and 120 postvaccination), the analyses included 103 participants (2D girls, n = 35; 3D girls, n = 38; 3D women, n = 30). Compared with the 716 participants in the original study [7] who did not participate at 120 months, 3D girls in this follow-up study were slightly younger (mean age 11.3 years vs 11.9 years at the first visit; P = .04); 3D women were less likely to be sexually active at recruitment (48.7% vs 68.1%; P = .03). For girls receiving 2 doses, there was no significant difference between the original trial cohort and those participating in this follow-up study (Table 1). At both the first study visit and at 120 months postvaccination, we did not observe a difference in 2D and 3D girls with regard to sociodemographics or sexual behavior (Supplementary Table A).
Figure 1.
Flow of participants throughout the study. Girls were aged 9–13 years at enrollment. Women were aged 16–26 years at enrollment. Abbreviation: HPV, human papillomavirus.
Table 1.
Comparison of Characteristics at First Study Visit/Vaccination Initiation of Participants Included in the Follow-up Study vs Participants Not Included in Follow-up
| Girls | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2D | 3D | 3D | |||||||
| Original Trial | Follow-up Study | P Value | Original Trial | Follow-up Study | P Value | Original Trial | Follow-up Study | P Value | |
| Age, mean (SD), y | 11.8 (1.3) | 11.9 (1.2) | .68 | 11.9 (1.3) | 11.3 (1.6) | .04 | 18.9 (2.7) | 18.2 (2.5) | .21 |
| Body mass index (kg/m2), mean (SD) | 19.6 (3.5) | 19.5 (3.3) | .90 | 19.9 (4.0) | 18.9 (2.7) | .18 | 23.0 (4.0) | 22.3 (2.3) | .51 |
| Weight (kg), mean (SD) | 46.9 (10.7) | 47.0 (11.5) | .94 | 48.4 (12.4) | 44.2 (11.2) | .08 | 62.2 (12.4) | 62.7 (8.7) | .42 |
| Postmenarchal, n (%) | 98 (44.1) | 19 (50) | .60 | 104 (47.1) | 13 (33.3) | .12 | 273 (100.0) | 37 (100.0) | … |
| Sexually active, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 186 (68.1) | 18 (48.7) | .03 | ||
| Age at sexual debut, mean (range), y | … | … | … | … | … | … | 16.7 (13–24) | 16.6 (14–19) | .96 |
| Number of sexual partners, mean (range) | … | … | … | … | … | … | 2.1 (1–4) | 1.7 (1–4) | .97 |
Abbreviation: 2D, 2 doses; 3D, 3 doses; SD, standard deviation.
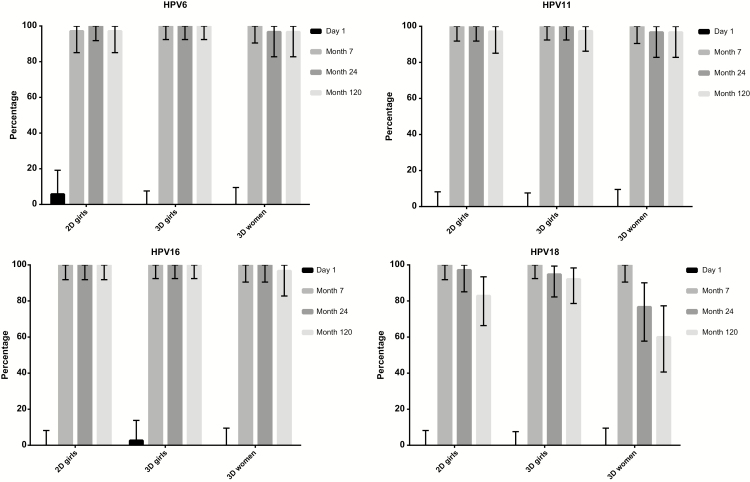
At 120 months postvaccination, cLIA seropositivity rates were above 95% for all HPV vaccine types and all schedules, except HPV18, which had the lowest seropositivity observed among 3D women (60.0%; 95% CI, 40.6%–77.3%; Figure 2). At 120 months, 2D and 3D girls had noninferior GMTs for all HPV vaccine types compared with 3D women. GMTs for 2D girls were noninferior at 120 months postvaccination compared with 3D girls for HPV6, 11, and 16 but not for HPV18 (Table 2). Trends in GMTs and seropositivity were comparable for the TIgG and the cLIA (Supplementary Table B and Supplementary Figure 1).
Figure 2.
Seropositivity rates as measured by competitive Luminex immunoassay at different time points. Seropositivity rates of human papillomavirus 6/11/16/18 among 2-(2D) and 3-dose (3D) girls and 3D women up to 120 months postvaccination. Abbreviation: HPV, human papillomavirus.
Table 2.
Summary of Competitive Luminex Immunoassay Geometric Mean Titers and Ratios at Day 1, Month 7, Month 24, and Month 120
| Girls | Women | GMT Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2D | 3D | 3D | 2D Girls/3D Women | 2D Girls/3D Girls | 3D Girls/3D Women | ||||
| No. | GMT (95% CI) | No. | GMT (95% CI) | No. | GMT (95% CI) | Ratio (95%CI) | Ratio (95%CI) | Ratio (95%CI) | |
| Day 1 | |||||||||
| HPV6 | 35 | 4 (3–5) | 38 | 4 (4–4) | 30 | 4a | … | … | … |
| HPV11 | 35 | 4a | 38 | 4a | 30 | 4 (4–5) | … | … | … |
| HPV16 | 35 | 6a | 38 | 6 (5–6) | 30 | 6a | … | … | … |
| HPV18 | 35 | 5a | 38 | 5a | 30 | 5a | … | … | … |
| Month 7 | |||||||||
| HPV6 | 35 | 1941 (1059–3559) | 38 | 2229 (1542–3219) | 30 | 1081 (631–1852) | 1.80 (0.80–4.03) | 0.87 (0.44–1.73) | 2.06 (1.11–3.83) |
| HPV11 | 35 | 2325 (1711–3160) | 38 | 2256 (1655–3075) | 30 | 1705 (1134-2563) | 1.36 (0.83–2.23) | 1.03 (0.67–1.58) | 1.32 (0.81–2.17) |
| HPV16 | 35 | 5804 (3397–9918) | 38 | 7721 (5237–11 384) | 30 | 3889 (2246–6736) | 1.49 (0.70–3.18) | 0.75 (0.40–1.43) | 1.99 (1.04–3.77) |
| HPV18 | 35 | 1496 (1041–2147) | 38 | 1995 (1318–3020) | 30 | 718 (461–1117) | 2.08 (1.20–3.63) | 0.75 (0.43–1.29) | 2.78 (1.53–5.06) |
| Month 24 | |||||||||
| HPV6 | 35 | 298 (202–439) | 38 | 313 (237–412) | 30 | 205 (153–274) | 1.46 (0.89–2.38) | 0.95 (0.60–1.51) | 1.53 (1.03–2.27) |
| HPV11 | 35 | 376 (277–510) | 38 | 395 (294–530) | 30 | 310 (205–467) | 1.21 (0.74–1.99) | 0.95 (0.63–1.44) | 1.28 (0.79–2.07) |
| HPV16 | 35 | 1561 (1118–2179) | 38 | 1472 (1063–2038) | 30 | 978 (675–1418) | 1.60 (0.98–2.60) | 1.06 (0.67–1.68) | 1.50 (0.93–2.44) |
| HPV18 | 35 | 188 (125-282) | 38 | 315 (202–489) | 30 | 90 (54–150) | 2.08 (1.11–3.90) | 0.60 (0.33–1.08) | 3.48 (1.80–6.71) |
| Month 120 | |||||||||
| HPV6 | 35 | 154 (109–217) | 38 | 164 (126–213) | 30 | 111 (80–155) | 1.39 (0.86–2.23) | 0.94 (0.62–1.43) | 1.48 (0.98–2.22) |
| HPV11 | 35 | 133 (91–193) | 38 | 148 (109–202) | 30 | 134 (89–201) | .99 (0.58–1.71) | 0.90 (0.56–1.44) | 1.10 (0.68–1.82) |
| HPV16 | 35 | 692 (492–973) | 38 | 571 (416–784) | 30 | 430 (264–699) | 1.61 (0.91–2.85) | 1.21 (0.77–1.92) | 1.33 (0.77–2.30) |
| HPV18 | 35 | 74 (47–118) | 38 | 103 (67–160) | 30 | 37 (21–65) | 2.02 (0.99–4.10) | 0.72 (0.38–1.34) | 2.82 (1.42–5.58) |
Abbreviations: 2D, 2 doses; 3D, 3 doses; CI, confidence interval; GMT, geometric mean titer; HPV, human papillomavirus.
aAll responses for this HPV type in this group were below the detection limit.
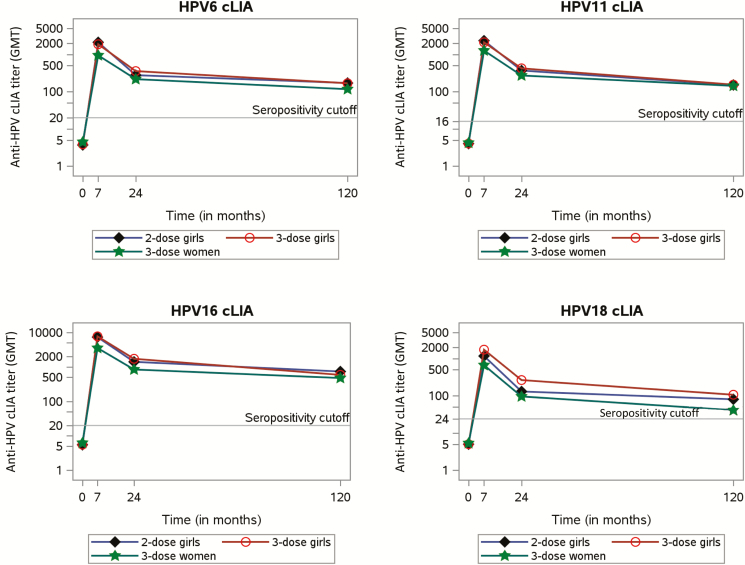
Between 24 and 120 months, there was a statistically significant reduction in GMTs for all HPV types for all groups (all P values < .001; Figure 3). We did not observe a significant difference in the development of GMT over time between the different study groups. The greatest rate of decline was found for HPV18 in 3D girls, log difference 1.11 (95% CI, 0.94–1.29), and the smallest decline was found among 3D women for HPV6, log difference 0.61 (95% CI, 0.32–0.91; Table 3). Trends were comparable for the TIgG and the cLIA (data not shown).
Figure 3.
Kinetics of competitive Luminex immunoassay antibody titers over time. Geometric mean titers for 2- and 3-dose girls and 3-dose women up to 120 months postvaccination. Abbreviations: cLIA, competitive Luminex immunoassay; GMT, geometric mean titer; HPV, human papillomavirus.
Table 3.
Difference in Competitive Luminex Immunoassay Geometric Mean Titers Between 24 and 120 Months Postvaccination as Estimated by a Linear Mixed Model
| Girls | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2D | 3D | 3D | |||||||
| Log Difference (95% CI) | P Value | Log Difference (95% CI) | P Value | Log Difference (95% CI) | P Value | P Value for Difference of Differences, 2D Girls vs 3D Women | P Value for Difference of Differences, 3D Girls vs 3D Women | P Value for Difference of Differences, 2D Girls vs 3D Girls | |
| HPV6 | 0.66 (0.39–0.93) | <.001 | 0.65 (0.51–0.78) | <.001 | 0.61 (0.32–0.91) | .002 | .90 | .94 | .92 |
| HPV11 | 1.04 (0.89–1.19) | <.001 | 0.98 (0.81–1.15) | <.001 | 0.84 (0.48–1.20) | <.001 | .20 | .36 | .59 |
| HPV16 | 0.81 (0.61–1.02) | <.001 | 0.95 (0.79–1.11) | <.001 | 0.82 (0.43–1.22) | .002 | .98 | .50 | .30 |
| HPV18 | 0.93 (0.65–1.21) | <.001 | 1.11 (0.94–1.29) | <.001 | 0.90 (0.45–1.35) | .003 | .96 | .44 | .26 |
Abbreviations: 2D, 2 doses; 3D, 3 doses; CI, confidence interval; HPV, human papillomavirus.
Although we observed declining antibody levels for all HPV types and all schedules, we did not observe a difference in the decay of antibody levels over time for the different schedules, since all interaction terms between time since first dose and schedule were nonsignificant (Supplementary Table C). Trends were comparable for the TIgG and the cLIA (data not shown).
DISCUSSION
At 120 months after their first dose of the HPV vaccine, 2D girls showed noninferior GMTs compared with 3D women, in whom efficacy had been established for all HPV vaccine types. Also, GMTs in 2D girls were noninferior to those in 3D girls, except for HPV18 when measured with cLIA. Measured with cLIA, seropositivity for all types was above 95% in all groups, except for HPV18. We did not observe a significant difference in the decline of antibody titers over time between 2D and 3D girls or between either 2D or 3D girls and 3D women. These data provide further reassurance in support of the World Health Organization recommendation for 2D schedules [6].
Seropositivity rates for HPV18 decreased over time and were significantly lower for 3D women compared with 2D or 3D girls when measured with the cLIA. The cLIA is known to be less sensitive for HPV18 than other assays such as the pseudovirus neutralizing antibody assay or the TIgG immunoassay [19]. Because of the great efficacy of the HPV vaccines, it has been impossible to distinguish a correlate of protection to date. Neutralizing antibodies are thought to be the principal mechanism of protection [20]. However, factors other than antibody levels might influence protection [21], especially in the longer term. Given the lack of a correlate of protection, the clinical value of our lower seropositivity rates cannot be defined within our study. While the lower seropositivity rates over time for HPV18 are in line with previous findings, this seemingly weaker antibody response has not been reflected in inferior protection against HPV-associated diseases [22, 23]. Joura et al showed declining seropositivity of up to 60% 44 months after vaccination in women, but efficacy at that time was still 98.4% (95% CI, 90.5%–100.0%) against HPV18-related cervical intraepithelial neoplasia/adenocarcinoma in situ of any grade [23]. In our study, when measured with the TIgG, the seropositivity in 3D women (87.0%; 95% CI, 78.8%–95.1%) was still lower compared with girls (2D: 94.7%; 95% CI, 87.5%–100.0% and 3D: 92.3%; 95% CI, 83.7%–100.0%), but this was no longer significantly different.
We showed that GMTs for both 2D and 3D girls were statistically noninferior to those for 3D women up to 120 months after initiation of 4vHPV vaccination. The original approval of HPV vaccines for girls was based on noninferior immune responses compared with women, with the assumption that immunogenicity efficacy would also be comparable [3]. Our findings confirm the durability of the immune response after 2D of 4vHPV vaccine for at least 10 years. Recently, effectiveness of 3D of the 4vHPV vaccine against CIN2+ vulvar and vaginal cancer to at least 10 years with indications for up to 12 years was shown in the Nordic countries among women who participated in the FUTURE II study and were vaccinated between age 15 and 26 years [24]. Combining these recent results with the noninferior immunogenicity observed in our study, we can assume that girls vaccinated with the 4vHPV vaccine will be well protected for more than a decade.
We observed noninferior antibody titers for all HPV vaccine types in 2D girls compared with 3D girls up to 120 months when measured with the TIgG and for all types, except HPV18, when measured with the cLIA. It is not known how the lower antibody titers for HPV18 after 2D would impact effectiveness, given the current absence of a correlate of antibody levels required for protection from HPV infection. A recent study from India also showed noninferior antibody responses comparing 2D and 3D recipients within their own age group (15–18 years) up to 36 months [25]. Also, continued responses were measured in this study up to 48 months postvaccination. In addition, in both arms of that study, no persistent infections with any of the vaccine types were found up to 7 years postvaccination, indicating comparable effectiveness [26].
An important limitation of our study was the small size of the study groups compared with the number of women in the original trial. However, the study had sufficient statistical power to demonstrate noninferiority at 120 months. In addition, comparing those participants who did or did not participate in the follow-up, we did not find remarkable differences in participant characteristics among the girls and expect limited impact of these small differences on the persistence of antibodies. The 3D women in the follow-up study were less likely to be sexually active (48.7% vs 68.1%) at the start of the study compared with the women who were included in the original trial but did not participate at 120 months. This could have influenced the antibody titers because the 3D women included in this follow-up study were less likely to be previously exposed. Because the antibody titers for the different time points were tested at different times, we cannot exclude the possibility of slight variability in the observed antibody titers. However, the observed kinetics of antibody titers indicate no important or unexpected variability.
In conclusion, this is the first study to show the continued antibody responses of 2D compared with 3D of HPV vaccine with follow-up to 10 years. The 2D schedule of the 4vHPV vaccine is highly immunogenic, and antibody responses persisted up to 10 years postvaccination.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. Merck & Co, Inc, Kenilworth, New Jersey, conducted the antibody assays at no cost to the study.
Financial support. The Michael Smith Foundation for Health Research, the Canadian Immunization Research Network, and the Ministries of Health of the provinces of British Columbia, Nova Scotia, and Quebec provided the funding for this project.
Potential conflicts of interest. R. D. reports grants from the Canadian Immunization Research Network and the Michael Smith Foundation of Health Research outside of the submitted work. S. R. M. D. reports a contract for a clinical trial funded by Merck & Co Inc outside of the submitted work titled, “A Phase III Clinical Trial to Study the Tolerability and Immunogenicity of a 2 Dose Regimen of V503, a Multivalent Human Papillomavirus (HPV) L1 Virus-Like Particle (VLP) Vaccine, Administered in Preadolescents and Adolescents (9 to 14 year olds) With a Comparison to Young Women (16 to 26 year olds).” C. S. received funding from Merck & Co outside of the submitted work during the conduct of the study. D. C. received personal fees from Hologic outside of the submitted work. V. G. reports a grant from the Bill and Melinda Gates Foundation outside of the submitted work during the conduct of the study. S. M. received grants and personal fees from GlaxoSmithKline and Pfizer and personal fees from Merck & Co and Sanofi Pasteur outside of the submitted work. M. K. reports grants from Roche and Hologic outside of the submitted work. D. M. reports grants from Merck, GlaxoSmithKline, Novartis, and the Canadian Institutes of Health Research outside of the submitted work. M. S. reports grants from GlaxoSmithKline and VBI Vaccines outside of the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Syrjänen K, Hakama M, Saarikoski S, et al. . Prevalence, incidence, and estimated life-time risk of cervical human papillomavirus infections in a nonselected Finnish female population. Sex Transm Dis 1990; 17:15–9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2154865. Accessed 13 May 2019. [PubMed] [Google Scholar]
- 2. de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 2017; 141:664–70. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28369882. Accessed 13 May 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. IARC HPV Working Group. Primary end-points for prophylactic HPV vaccine trials International Agency for Research on Cancer, 2014. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26468561. Accessed 9 July 2019. [Google Scholar]
- 4. The Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–27. Available at: http://www.nejm.org/doi/abs/10.1056/NEJMoa061741. Accessed 14 August 2019. [DOI] [PubMed] [Google Scholar]
- 5. Harper DM, Franco EL, Wheeler CM, et al. ; HPV Vaccine Study Group Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247–55. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization. Human papillomavirus vaccines: WHO position paper, October 2014–recommendations. Vaccine 2015; 33:4383–4. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25510390. Accessed 12 July 2019. [DOI] [PubMed] [Google Scholar]
- 7. Dobson SR, McNeil S, Dionne M, et al. . Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309:1793–802. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23632723. Accessed 28 February 2019. [DOI] [PubMed] [Google Scholar]
- 8. Romanowski B, Schwarz TF, Ferguson LM, et al. . Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared with the licensed 3-dose schedule: results from a randomized study. Hum Vaccin 2011; 7:1374–86. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22048171. Accessed 14 August 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Romanowski B, Schwarz TF, Ferguson LM, et al. . Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: results from a randomized study. Hum Vaccin Immunother 2014; 10:1155–65. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24576907. Accessed 14 August 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donken R, Bogaards JA, van der Klis FR, Meijer CJ, de Melker HE. An exploration of individual- and population-level impact of the 2-dose HPV vaccination schedule in pre-adolescent girls. Hum Vaccin Immunother 2016; 12:1381–93. Available at: http://dx.doi.org/10.1080/21645515.2016.1160978. Accessed 8 March 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ogilvie G, Sauvageau C, Dionne M, et al. . Immunogenicity of 2 vs 3 doses of the quadrivalent human papillomavirus vaccine in girls aged 9 to 13 years after 60 months. JAMA 2017; 317:1687–8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28444267. Accessed 28 February 2019. [DOI] [PubMed] [Google Scholar]
- 12. Donken R, Knol MJ, Bogaards JA, van der Klis FR, Meijer CJ, de Melker HE. Inconclusive evidence for non-inferior immunogenicity of two- compared with three-dose HPV immunization schedules in preadolescent girls: a systematic review and meta-analysis. J Infect 2015; 71:61–73. Available at: https://www.sciencedirect.com/science/article/pii/S016344531500064X?via%3Dihub. Accessed 28 February 2019. [DOI] [PubMed] [Google Scholar]
- 13. Krajden M, Cook D, Yu A, et al. . Assessment of HPV 16 and HPV 18 antibody responses by pseudovirus neutralization, Merck cLIA and Merck total IgG LIA immunoassays in a reduced dosage quadrivalent HPV vaccine trial. Vaccine 2014; 32:624–30. Available at: https://www.sciencedirect.com/science/article/pii/S0264410X13012346?via%3Dihub. Accessed 28 February 2019. [DOI] [PubMed] [Google Scholar]
- 14. Dias D, Van Doren J, Schlottmann S, et al. . Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol 2005; 12:959–69. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16085914. Accessed 26 March 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Opalka D, Matys K, Bojczuk P, et al. . Multiplexed serologic assay for nine anogenital human papillomavirus types. Clin Vaccine Immunol 2010; 17:818–27. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20237197. Accessed 26 June 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reisinger KS, Block SL, Lazcano-Ponce E, et al. . Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J 2007; 26:201–9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17484215. Accessed 11 April 2019. [DOI] [PubMed] [Google Scholar]
- 17. Kaji AH, Lewis RJ. Noninferiority trials. JAMA 2015; 313:2371 Available at: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2015.6645. Accessed 14 August 2019. [DOI] [PubMed] [Google Scholar]
- 18. Wang WW, Mehrotra DV, Chan IS, Heyse JF. Statistical considerations for noninferiority/equivalence trials in vaccine development. J Biopharm Stat 2006; 16:429–41. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16892905. Accessed 14 August 2019. [DOI] [PubMed] [Google Scholar]
- 19. Brown DR, Garland SM, Ferris DG, et al. . The humoral response to Gardasil over four years as defined by total IgG and competitive Luminex immunoassay. Hum Vaccin 2011; 7:230–8. Available at: http://www.tandfonline.com/doi/abs/10.4161/hv.7.2.13948. Accessed 14 August 2019. [DOI] [PubMed] [Google Scholar]
- 20. Pinto LA, Dillner J, Beddows S, Unger ER. Immunogenicity of HPV prophylactic vaccines: serology assays and their use in HPV vaccine evaluation and development. Vaccine 2018; 36:4792–9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/29361344. Accessed 6 March 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stanley MA, Sudenga SL, Giuliano AR. Alternative dosage schedules with HPV virus-like particle vaccines. Expert Rev Vaccines 2014; 13:1027–38. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25001893. Accessed 14 August 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Villa LL, Costa RL, Petta CA, et al. . High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006; 95:1459–66. Available at: http://www.nature.com/articles/6603469. Accessed 14 August 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joura EA, Kjaer SK, Wheeler CM, et al. . HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine 2008; 26:6844–51. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18930097. Accessed 14 August 2019. [DOI] [PubMed] [Google Scholar]
- 24. Kjaer SK, Nygård M, Dillner J, et al. . A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis 2018; 66:339–45. Available at: http://academic.oup.com/cid/article/66/3/339/4283361. Accessed 14 August 2019. [DOI] [PubMed] [Google Scholar]
- 25. Bhatla N, Nene BM, Joshi S, et al. ; Indian HPV Vaccine Study Group Are two doses of human papillomavirus vaccine sufficient for girls aged 15–18 years? Results from a cohort study in India. Papillomavirus Res 2018; 5:163–71. Available at: http://creativecommons.org/licenses/BY-NC-ND/4.0/. Accessed 14 August 2019. [DOI] [PMC free article] [PubMed]
- 26. Sankaranarayanan R, Joshi S, Muwonge R, et al. ; Indian HPV Vaccine Study Group Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine 2018; 36:4783–91. Available at: https://www.sciencedirect.com/science/article/pii/S0264410X1830286X?via%3Dihub. Accessed 7 March 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.