Figure 4.
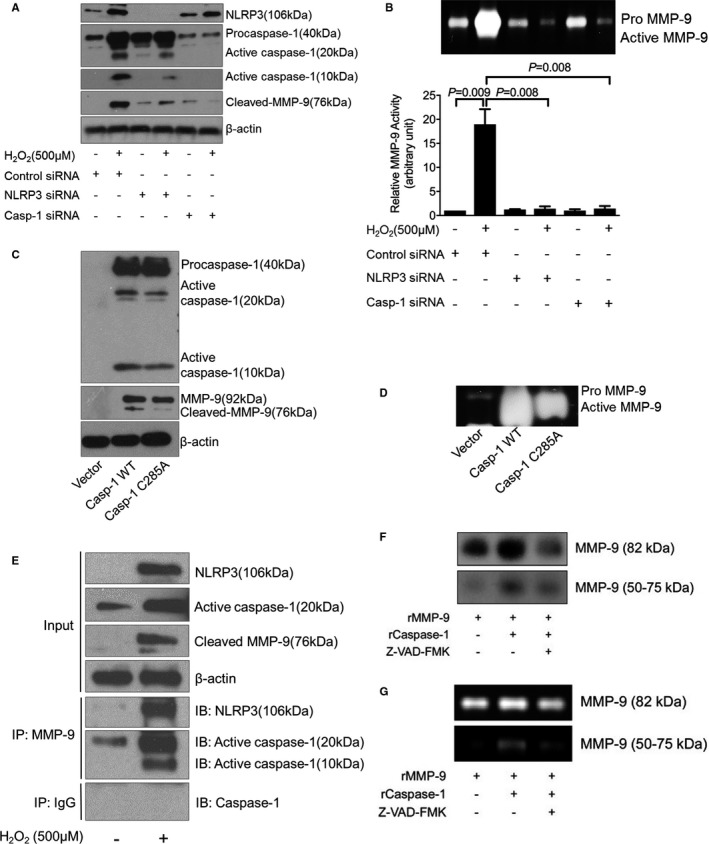
Activation of matrix metallopeptidase 9 (MMP‐9) by the nucleotide‐binding oligomerization domain–like receptor pyrin domain containing 3 (NLRP3)–caspase‐1 cascade in human THP‐1 macrophages. A, Representative Western blot results showing that silencing NLRP3 or caspase‐1 with siRNA prevented H2O2‐induced MMP‐9 activation. B, In‐gel zymography results showing that silencing NLRP3 or caspase‐1 with siRNA reduced MMP‐9 activity. C, Representative Western blot results showing that the overexpression of capase‐1 increased the cleavage/activation of MMP‐9 but that the overexpression of a dominant‐negative caspase‐1 mutant (Casp‐1 S285A) failed to induce MMP‐9 cleavage/activation. D, In‐gel zymography results showing that the overexpression of capase‐1 increased the activity of MMP‐9 but that the overexpression of a dominant‐negative caspase‐1 mutant (Casp‐1 S285A) reduced MMP‐9 activity. E, Results of coimmunoprecipitation analysis showing evidence of an interaction between NLRP3/capase‐1 with MMP‐9. F, Representative cleavage assay results showing that recombinant caspase‐1 (rCaspase‐1) directly cleaves recombinant MMP‐9 (rMMP‐9). Z‐VAD‐FMK, caspase‐1 inhibitor. G, The cleaved MMP‐9 fragment (50‐75 kDa) exhibiting gelatin digestive ability. Data are presented as mean±SEM. One‐way ANOVA with the post hoc Dunnett T3 test was used for pairwise comparisons. WT indicates wild‐type.
