Figure 2. DYRK1A missense mutation affect catalytic pocket of kinase domain.
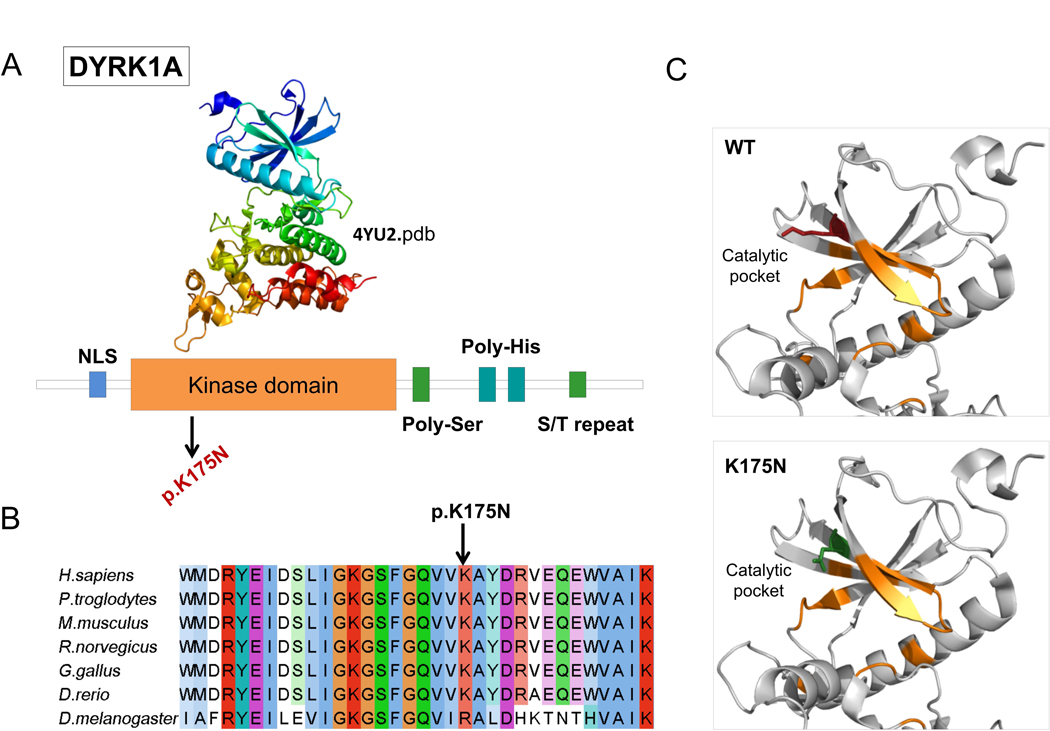
A) Domain architecture of DYRK1A. Protein sequence presents regions biased toward polar (serine and threonine) and aromatic (histidine residues). NLS = nuclear localization signal. B) DYRK1A p.Lys175 and neighboring residues are conserved among orthologous sequences. Amino acids are colored by conservation, according to ClustalX color code. C) DYRK1A p. Lys175Asn variant and wild type residues are mapped to kinase domain structure (4yu2.pdb, chain A). Residues involved in nucleotide binding are represented in orange sticks, wild-type lysine (K) in red and asparagine (N) in green.
