Abstract
Background
Epidemiologic studies, including trials, suggest an association between potassium intake and blood pressure (BP). However, the strength and shape of this relationship is uncertain.
Methods and Results
We performed a meta‐analysis to explore the dose‐response relationship between potassium supplementation and BP in randomized‐controlled trials with a duration ≥4 weeks using the recently developed 1‐stage cubic spline regression model. This model allows use of trials with at least 2 exposure categories. We identified 32 eligible trials. Most were conducted in adults with hypertension using a crossover design and potassium supplementation doses that ranged from 30 to 140 mmol/d. We observed a U‐shaped relationship between 24‐hour active and control arm differences in potassium excretion and BP levels, with weakening of the BP reduction effect above differences of 30 mmol/d and a BP increase above differences ≈80 mmol/d. Achieved potassium excretion analysis also identified a U‐shaped relationship. The BP‐lowering effects of potassium supplementation were stronger in participants with hypertension and at higher levels of sodium intake. The BP increase with high potassium excretion was noted in participants with antihypertensive drug‐treated hypertension but not in their untreated counterparts.
Conclusions
We identified a nonlinear relationship between potassium intake and both systolic and diastolic BP, although estimates for BP effects of high potassium intakes should be interpreted with caution because of limited availability of trials. Our findings indicate an adequate intake of potassium is desirable to achieve a lower BP level but suggest excessive potassium supplementation should be avoided, particularly in specific subgroups.
Keywords: blood pressure, dietary supplement, dose‐response meta‐analysis, potassium
Subject Categories: Diet and Nutrition, Meta Analysis, Blood Pressure, Primary Prevention
Nonstandard Abbreviations and Acronyms
- BP
blood pressure
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- RCT
randomized controlled trial
- RoB
risk of bias
- SBP
systolic blood pressure
Clinical Perspective
What Is New?
Use of the new “1‐stage” natural cubic spline model allowed, for the first time, pooling of experience in 2‐arm randomized controlled trials to characterize the dose‐response relationship between potassium supplementation and blood pressure (BP).
Results of this dose‐response meta‐analysis suggested a nonlinear relationship that included BP reduction but also indicated that both low and high potassium intake may result in an increased level of BP, particularly but not exclusively in participants with hypertension.
What Are the Clinical Implications?
There seems to be a U‐shaped relationship between potassium intake and BP, which might explain reports of deleterious cardiovascular disease outcomes at low and high intakes of potassium, and suggests an optimal BP‐lowering range for potassium intake.
Modification of dietary factors may affect the risk of cardiovascular diseases (CVDs).1, 2, 3 A primary mechanism of action is through lowering blood pressure (BP), the most important major modifiable risk factor for CVD.4, 5, 6 Both a lower sodium and a higher potassium intake have been associated with lowering of BP and a reduction in CVD.7, 8, 9, 10 The role of these elements in BP control has been studied extensively in laboratory and epidemiological studies.5, 11, 12, 13 In particular, experimental human studies (ie, randomized controlled trials [RCTs]) suggest that potassium supplementation may decrease BP,14, 15, 16, 17 particularly in adults with hypertension.12 However, an accurate assessment of the potassium‐BP dose‐response relationship has not been possible because of a lack of biostatistical models to conduct flexible, curvilinear modeling of RCTs with only 2 levels of exposure (placebo and potassium supplementation).12, 18, 19 This has also hampered the use of evidence on the BP effects of potassium in recent risk assessments of adequate potassium intake performed by the European Food Safety Authority and the US National Academy of Medicine.13, 19, 20 These assessments have therefore focused on outcomes, such as stroke21 and other CVD events,14, 19 although this evidence is limited by availability of only a relatively small number of studies that have used an observational design. In contrast, many RCTs have been conducted for estimation of the effect of potassium on BP. Some evidence has accrued from observational studies suggesting that a high potassium intake may increase the risk of hypertension,22 stroke,21 and CVD mortality.23, 24 This has resulted in some concern about the potential for long‐term adverse effects of a high potassium intake in the general population.23, 24, 25, 26, 27, 28, 29
In this review, we aimed to assess the dose‐response relationship between potassium intake and BP on the basis of use of a new biostatistical method,30 which allowed us to use experimental studies based on comparisons of 2 levels of potassium exposure, as is typical in most RCTs. In addition, we sought to compare the results of our dose‐response meta‐analysis with corresponding assessments generated using conventional meta‐analysis analytic techniques based on the assumption of a linear association between potassium intake and BP.
Methods
The authors declare that all supporting data are available within the article and its online supplementary files.
Literature Search
We conducted a literature search for articles published on or before March 14, 2020, using the PubMed database, with no language restriction. The research question was configured according to the Population, Exposure, Comparator(s), Outcomes, and Study Design statement and used the search terms “potassium” and “blood pressure.”31 Details of the search strategy are provided in Table S1. Reference lists were screened to identify additional publications.
A study was considered eligible if: (1) it was performed in participants with hypertension (apart from secondary hypertension) or without hypertension; (2) exposure to potassium was assessed through use of either dietary questionnaires or urinary measurements; (3) the outcome of interest was systolic BP (SBP), diastolic BP (DBP), or both; (4) an experimental design and a minimum intervention duration of 4 weeks had been used, to ensure biological effect of the intervention, increase comparability with long‐term habitual potassium intake, and provide consistently with recent systematic reviews14, 18, 19; (5) the intervention was performed using potassium‐containing supplements, and not through dietary modification only or by administration of mixed interventions with other active components; and (6) measurements of urinary sodium and potassium excretion obtained before and after potassium supplementation were available. The trial results were imported into Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia; http://www.covidence.org) for further assessment and data extraction. At least 2 authors reviewed all titles and abstracts independently. If they disagreed, the final decision was reached by a majority decision with the help of a third author.
Risk of Bias Assessment
We conducted an independent assessment of study quality using the risk of bias (RoB) assessment tool (2.0). The following 6 RoB domains were considered: (1) randomization process errors; (2) deviations from the intended interventions; (3) missing outcome data; (4) systematic errors in measurement of the outcome; (5) bias in selection of the reported result. In addition, we included an evaluation of the (6) RoB related to use of a crossover study design, assessing the use of a washout period and whether the trial duration was at least 4 weeks. Each domain could be characterized as having a low RoB, some concerns, or a high RoB. A study was assigned an overall higher RoB if it was judged to be at higher risk for at least 1 domain, and an intermediate RoB when some concern existed for at least 1 of domains 1, 2, and 6, or for ≥2 domains 3 to 5.
Data Extraction
For each eligible study, the following data were extracted independently by 2 of the authors (M.I.K., T.F.) and confirmed by a third author (D.T.): first author name, publication year, country, duration of potassium intervention phase, number of participants and their characteristics (sex, age, hypertensive status, use of antihypertensive medication), study design, presence and duration of a washout period, modality of BP measurement, type and quantity of the potassium supplements, baseline and achieved potassium excretion level, sodium excretion at baseline and after the intervention, modification of sodium intake, and summary statistics of SBP and DBP levels (mean level in each group, active and control, for crossover studies or mean difference for parallel studies along with SD/SE).
Statistical Analysis
We performed a meta‐analysis of SBP and DBP weighted mean differences before and after potassium supplementation for each study and for the relevant subgroups using a “1‐stage” natural cubic spline regression model on the basis of a random effects model,32 assessing heterogeneity with the I2 statistic.33 The 1‐stage method, consisting of a weighted mixed effects model, was recently developed30 and used in dose‐response meta‐analysis,34, 35 and it allowed us to make inferences about the average dose‐response relationship between changes in potassium excretion attributable to supplementation or overall potassium excretion at the end of the trial and changes in SBP and DBP levels. The 1‐stage approach allowed us to include trials based on 2 levels of exposure, as was the case for most of the trials included in our study. Having no specific parametric assumptions about the shape of the association, we used restricted cubic splines of potassium with 3 knots at fixed percentiles (10%, 50%, and 90%).36 For comparison, we also used a linear function to model potassium intake in relationship to level of BP. Estimates of the parameters were obtained using restricted maximum likelihood.30, 36
We defined the mean difference in potassium excretion between the arms of each RCT as the difference between the values of potassium excretion at the end of the trial and the ones at baseline in each arm. Likewise, we defined the mean difference in BP following the intervention as the difference for SBP and DBP at the end of the trial minus the corresponding baseline value.
In addition to the main analysis, we conducted stratified analyses based on study design (parallel versus crossover), hypertension status, use of antihypertensive medication (excluding normotensives), baseline potassium excretion (<75 and ≥75 mmol/d), position during BP measurement (supine, seated, standing, or other), type of BP measurement device (automatic or manual), baseline sodium excretion (<3, 3–4, or ≥4 g/d), and length of follow‐up (≥12 weeks). In sensitivity analyses, we excluded trials at high risk for bias. We also reran the main analysis repeatedly, each time without one of the studies, to assess the missing study's influence on overall mean BP change, and we assessed the study‐specific dose‐response trends in comparison with the corresponding dose‐response meta‐analysis for all trials.
Publication bias was examined using funnel plots. We used Stata statistical software (Stata Corp, College Station, TX, 2019) for our data analysis, including the 1‐stage approach based on the drmeta command.37
Results
The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses literature search flowchart is presented in Figure 1. We retrieved 236 unique study articles, 144 of which were excluded on the basis of the article's title or abstract. Main reasons for exclusion were: nonexperimental design (including case reports), experimental studies where the intervention did not include potassium supplementation or where potassium was included in a mixed intervention with other active components, secondary hypertension, and animal and in vitro studies. Following full‐text review, we excluded 60 of the remaining 92 articles because they were review articles, were reports based on a potassium supplementation phase <4 weeks, did not report on urinary excretion of potassium or sodium, did not provide BP levels, were not based on a potassium supplementation trial, and were duplicate reports or detailed studies confined to children.
Figure 1. Flowchart of systematic literature search for trials published through March 14, 2020, that met the study inclusion and exclusion criteria.
The Table presents main characteristics of the 32 eligible trials in our meta‐analysis.38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70 The trials were published between 1982 and 2016. They included 1764 participants from Europe (N=17), America (N=7), Asia (N=4), Oceania (N=3), and Africa (N=1). All had been conducted in both sexes, with the exception of 2 that were restricted to women and 1 to men. Participant age ranged from 18 to 79 years, with mean values between 24 and 75 years. Nine trials used a parallel design, whereas 23 were crossover studies, with 5 of the latter including a washout period of 1 to 5 weeks. Most (N=27) were conducted in participants with hypertension, in 6 of which prior treatment with antihypertensive medication (mainly β blockers, thiazide, or calcium channel blockers) was continued during the trial, whereas 4 trials were restricted to participants without hypertension. BP was measured using an automatic device (n=15), a manual device (N=13), or both (N=4). Potassium was administered in the form of potassium chloride (N=28), citrate (N=6), carbonate (N=2), aspartate (N=1), and/or glucoronate (N=1) at potassium doses that generally ranged from 30 to 120 mmol/d. All the trials had estimates of 24‐hour potassium excretion in each study arm, both at baseline and at the end of the intervention. The achieved difference in potassium excretion at the end of the trial ranged from 17 to 131 mmol/d.
Table 1.
Characteristics of the 32 Trials Studied
| Reference | Year | Country | Duration of suppl. Phase | No. of Participants | Sex | Age, y (Mean) | Age, y (Range) | Design | Washout | Hypertension | Use of Anti‐hyp. medication | BP measure Device | BP Modality of Measurement | K Supplement | K Quantity | Modification of Na intake | Baseline uK | uK Suppl./uK Placebo | Achieved uK Difference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Barden 198640 (Gr1) (Gr2) |
1986 | Australia | 4 wk |
22 22 |
Women | 32 | 18–55 | Crossover | No | No | ··· | Automatic | Supine and standing | KCl | 80 | No |
49 58 |
107/52 132/50 |
55 82 |
| Berry 201039 | 2010 | United Kingdom | 6 wk | 48 | Both | 45 | 22–65 | Crossover | ≥5 wk | Yes | No | Automatic | Supine and ambulatory (24 h, awake and asleep) | K‐cit | 40 | No | 60 | 87/60 | 27 |
| Braschi 200838 | 2008 | United Kingdom | 6 wk |
90 56 (26+30)t 34c |
Both | 35 | 22–65 | Parallel | ··· | No | ··· | Automatic | Seated |
KCl K‐cit |
30 | No |
72t 78t 72c |
90/72 98/72 |
18 26 |
| Bulpitt 198541 | 1985 | United Kingdom | 12 wk |
33 14t 19c |
Both | 55 | ··· | Parallel | ··· | Yes | Yes | Manual and automatic | Mean of supine and standing | KCl | 64 | No |
72t 61c |
95/55 | 40 |
| Chalmers 198642 | 1986 | Australia | 4 wk |
24 13t 11c |
Both | 52 | ··· | Parallel | ··· | Yes | No | Automatic | Seated | KCl | 64 | No | 71 | 110/76 | 34 |
| Forrester 198843 | 1988 | Jamaica | 4 wk | 23 | Both | ··· | >18 | Crossover | No | Yes | Yes | Manual | Supine and standing | KCl | 48 | No | 43 | 67/43 | 24 |
| Fotherby 199244 | 1992 | United Kingdom | 4 wk | 18 | Both | 75 | 66–79 | Crossover | No | Yes | No | Manual and automatic | Supine, standing and ambulatory (24 h, day‐time and night‐time) | KCl | 60 | No | 63 | 99/60 | 39 |
| Franzoni 200545 | 2005 | Italy | 4 wk |
104 52t 52c |
Both | 52 | ··· | Parallel | ··· | Yes | No | Manual and automatic | Seated and ambulatory (24 h, daytime and nighttime) | K‐asp | 30 | No |
58t 55c |
82/58 | 24 |
| Gijsbers 201546 | 2015 | The Netherlands | 4 wk | 36 | Both | 66 | ··· | Crossover | No | Yes | No | Automatic | Seated and ambulatory (24 h, daytime and nighttime) | KCl | 38 | Yes ↑ | 49 | 118/55 | 63 |
| Graham 201447 | 2014 | United Kingdom | 6 wk | 40 | Both | 55 | 40–70 | Crossover | 2–4 wk | Yes | No | Automatic | Supine | KCl | 64 | No | 79 | 104/87 | 17 |
| Grimm 198848 | 1988 | Minnesota, United States | 12 wk |
198 148t 150c |
Men | 58 | 45–68 | Parallel | … | Yes | Yes | Manual | Seated | KCl | 96 | No |
82t 76c |
150/76 | 74 |
| Grobbee 198749 | 1987 | The Netherlands | 6 wk | 40 | Both | 24 | 18–28 | Crossover | No | Yes | No | Manual | Supine | KCl | 72 | No | 71 | 131/74 | 57 |
| Gu 200150 | 2001 | China | 12 wk |
150 75t 75c |
Both | 56 | ··· | Parallel | ··· | Yes | No | Manual | Seated | KCl | 60 | No |
36t 36c |
57/34 | 23 |
|
He 201070 KCl KHCO3 |
2010 | United Kingdom | 4 wk | 42 | Both | 51 | 18–75 | Crossover | No | Yes | No | Automatic | Seated and ambulatory (24 h, daytime and nighttime) |
KCl KHCO3 |
64 64 |
No | 80 |
122/77 125/77 |
45 48 |
| Kaplan 198551 | 1985 | Texas, United States | 6 wk | 16 | Both | 49 | 35–66 | Crossover | No | Yes | Yes | Manual | Supine | KCl | 60 | No | 46 | 82/36 | 46 |
| Kawano 199852 | 1998 | Japan | 4 wk | 55 | Both | ··· | 36–77 | Crossover | No | Yes | Yes | Manual and automatic | Supine and ambulatory (24 h, daytime and nighttime) | KCl | 64 | No | 42 | 96/54 | 42 |
| MacGregor 198253 | 1982 | England, United Kingdom | 4 wk | 23 | Both | 45 | 26–66 | Crossover | No | Yes | No | Automatic | Supine and standing | KCl | 60 | No | 56 | 118/62 | 56 |
| Matlou 198654 | 1986 | South Africa | 6 wk | 32 | Women | 51 | 34–62 | Crossover | No | Yes | No | Automatic | Seated | KCl | 65 | No | 62 | 114/52 | 62 |
| Matthensen 201255 | 2012 | Denmark | 4 wk | 21 | Both | 26 | 18–40 | Crossover | No | No | ··· | Automatic | Ambulatory | KCl | 100 | No | 76 | 168/76 | 92 |
| Miller 198756 | 1987 | Indiana, United States | 4 wk | 64 | Both | 42 | ··· | Crossover | No | No | ··· | Manual | Seated |
K‐cit K‐gluc |
60 | No | 59 | 82/59 | 23 |
| Overlack 198557 | 1985 | Germany | 8 wk | 17 | Both | 29 | 22–39 | Crossover | No | Yes | No | Manual | Supine and standing | KCl | 96 | No | 66 | 153/71 | 82 |
| Overlack 199158 | 1991 | Germany | 8 wk | 12 | Both | 37 | 25–59 | Crossover | No | Yes | No | Manual | Seated | K‐cit and KHCO3 | 120 | No | 74 | 167/62 | 105 |
|
Overlack 199559 KCl K‐cit |
1995 | Germany | 8 wk |
25 25 |
Both | 48 | 24–70 | Crossover | 4 wk | Yes | No | Automatic | Seated |
KCl K‐cit |
120 | No | 94 |
202/94 225/94 |
108 131 |
| Patki 199060 | 1990 | India | 8 wk | 37 | Both | 50 | ··· | Crossover | 2 wk | Yes | No | Manual | Supine and standing | KCl | 60 | No | 62 | 82/60 | 22 |
| Richards 198461 | 1984 | New Zealand | 4 wk | 12 | Both | ··· | 19–52 | Crossover | No | Yes | No | Automatic | Supine, standing and intra‐arterial 24‐h measure | KCl | 140 | Yes ↓ | 62 | 185/62 | 123 |
| Siani 198762 | 1987 | Italy | 15 wk |
37 18t 19c |
Both | 45 | 21–61 | Parallel | ··· | Yes | No | Manual | Supine and standing | KCl | 48 | No |
57t 62c |
87/57 | 30 |
|
Skrabal 198463 (Gr1) (Gr2) |
1984 | Austria | 4 wk |
21 9 12 |
Both |
32 45 |
21–46 28–69 |
Crossover | No | Yes |
No yes |
Manual | Supine, seated and standing | KCl | 40 | Yes ↓ |
80 65 |
117/80 82/65 |
38 17 |
| Smith 198564 | 1985 | England, United Kingdom | 4 wk | 20 | Both | 53 | 30–66 | Crossover | No | Yes | No | Automatic | Supine and standing | KCl | 64 | Yes ↓ | 72 | 117/67 | 50 |
| Sundar 198565 | 1985 | India | 4 wk |
50 25t 25c |
Both | 46 | ··· | Parallel | ··· | Yes | No | Manual | Supine | KCl | 60 | No |
57t 55c |
81/56 | 25 |
| Valdes 199166 | 1991 | South America (Chile) | 4 wk | 24 | Both | 50 | ··· | Crossover | No | Yes | No | Automatic | Supine and standing | KCl | 64 | No | 57 | 123/55 | 68 |
| Vongpatanasi 201667 | 2016 | Texas, United States | 4 wk | 30 | Both | 54 | ··· | Crossover | 1 wk | Yes | No | Automatic | Office, 24‐h average, daytime and nighttime |
K‐cit KCl |
40 | No | 58 |
84/58 95/58 |
26 37 |
| Whelton 199568, 69 | 1995 | North America | 4 wk |
353 178t 175c |
Both | 26 | 30–54 | Parallel | ··· | No | No | Manual | Seated | KCl | 60 | No | 59 | 97/55 | 42 |
Values of potassium levels are reported in mmol/d (only integers). BP indicates blood pressure; c, control group; Gr1, group 1; Gr2, group 2; K‐asp, potassium aspartate; K‐cit, potassium citrate; KCl, potassium chloride; K‐gluc, potassium gluconate; KHCO3, potassium bicarbonate; and t, treated group.
RoB assessment results are presented in Table S2, with reference to both single‐item evaluation and overall RoB. Overall, we judged only 2 of the trials as having a high RoB.43, 56
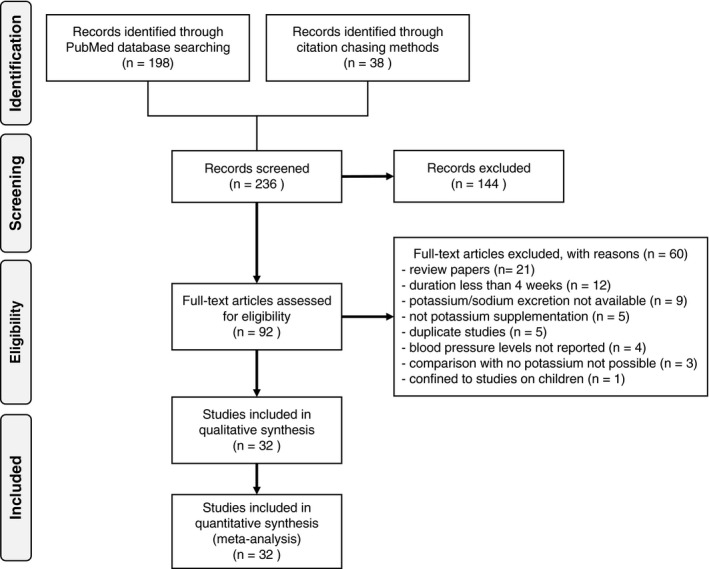
In the dose‐response meta‐analysis assessing effects of changes in potassium excretion between the control and supplemented groups on BP changes within each trial (Figure 2), we found that mean SBP and DBP levels decreased in the supplemented group with increasing differences in potassium excretion, up to a value of ≈30 mmol/d. At higher levels of supplementation, the decrease in BP was reduced, up to approximately a net difference in urinary potassium of 80 mmol/d. More substantial net differences in urinary potassium between the supplemented and unsupplemented participants resulted in an increase in both SBP and DBP. Increases of 30, 60, 90, and 120 mmol/d in net urinary potassium excretion differences between the supplemented and unsupplemented participants resulted in SBP changes of −3.3 (95% CI, −4.9 to −1.6), −2.0 (95% CI, −3.4 to −0.5), 1.1 (95% CI, −2.9 to 4.7), and 4.2 (95% CI, −2.3 to 10.6) mm Hg, respectively. For DBP, the corresponding changes were −2.3 (95% CI, −3.8 to −0.7), −1.3 (95% CI, −2.8 to 0.1), 0.86 (95% CI, −2.9 to 4.6), and 3.1 (95% CI, −3.5 to 9.7) mm Hg, respectively.
Figure 2. Dose‐response meta‐analysis of changes in systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels (as mm Hg), according to differences in potassium excretion between the treatment arms (potassium supplemented and control group) at the end of the trials.
All studies included (N=32). Spline curve (solid line) with 95% confidence limits (long dashed lines).
When we superimposed the average predicted mean difference in BP estimated according to a linear function into the dose‐response graph, it showed an inverse association between potassium supplementation and both SBP and DBP (Figure S1). A forest‐plot meta‐analysis comparing BP levels in the supplemented and referent groups identified a mean difference of −3.9 (95% CI, −5.2 to −2.6) and −2.4 (95% CI, −3.8 to −1.1) mm Hg for SBP and DBP, respectively (Figure S2).
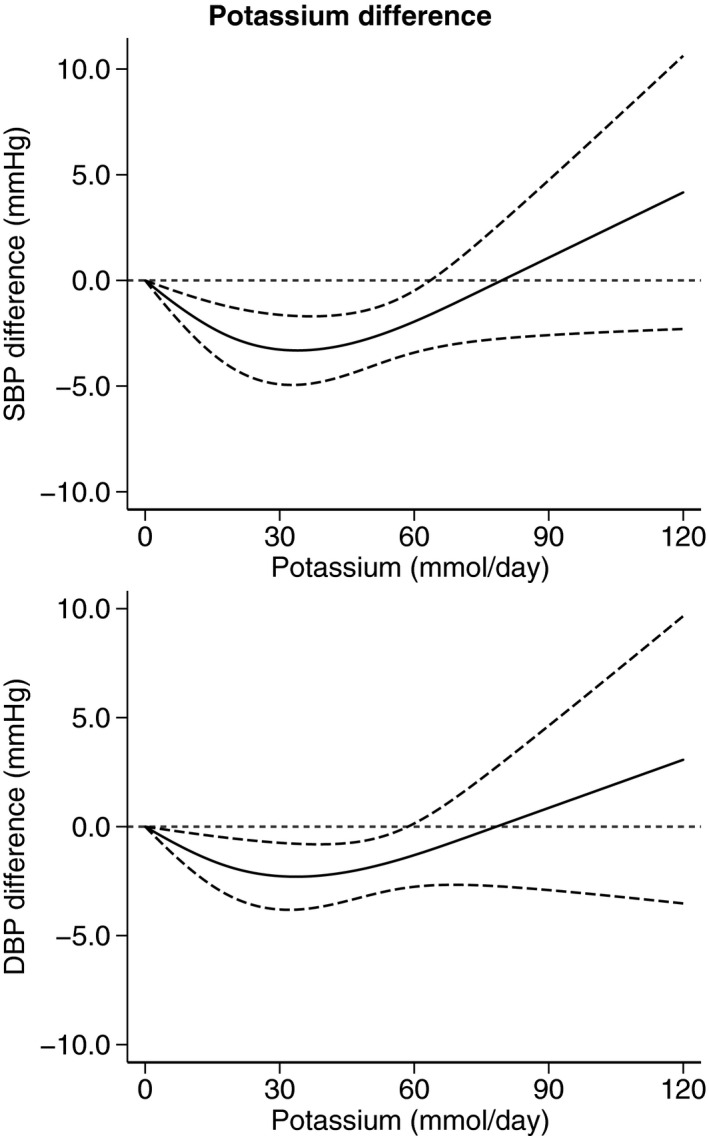
Figure 3 presents the BP difference in our dose‐response meta‐analysis on the basis of achieved potassium excretion at the end of the trial, using as a reference point a potassium excretion of 90 mmol/d (3500 mg/d). The SBP and DBP change remained constant in the range of 90 to 150 mmol/d of achieved potassium excretion. Below these ranges of achieved potassium excretion, the intervention effects on BP were unfavorable, and a weak BP increase also appeared to occur at >150 mmol/d. A potassium excretion of 30, 60, 120, 150, and 180 mmol/d resulted in SBP changes of 9.1 (95% CI, 4.6–13.5), 3.9 (95% CI, 2.1–5.8), −0.9 (95% CI, −1.6 to −0.2), −0.2 (95% CI, −2.2 to 1.8), and 0.7 (95% CI, −2.9 to 4.2) mm Hg, respectively, compared with the SBP associated with an excretion of 90 mmol/d. The corresponding DBP changes were 5.3 (95% CI, 0.9–9.7), 2.3 (95% CI, 0.5–4.1), −0.4 (95% CI, −1.5 to 0.7), 0.2 (95% CI, −3.0 to −3.3), and 0.8 (95% CI, −4.6 to 6.2). Again, as for the analysis based on the BP effects of difference in potassium excretion between the 2 exposures, the predicted mean SBP and DBP difference on the basis of a linear regression function shows an inverse association with achieved potassium intake (Figure S1).
Figure 3. Dose‐response meta‐analysis of changes in systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels (as mm Hg), according to achieved potassium excretion levels between arms (potassium supplemented and control group) at the end of the trials.
All studies included (N=32). Spline curve (solid line) with 95% confidence limits (long dashed lines).
When we excluded the studies deemed to have a high RoB, the dose‐response analysis yielded similar results of that generated using the entire data set (Figures S3 and S4). We repeated the main analysis after systematically excluding each study in turn from the meta‐analysis, and no appreciable variation to the overall mean change in BP was noted (Figures S5 and S6). Similarly, a sensitivity analysis showing variation of the shape across studies identified study‐specific trends that were generally similar to the overall dose‐response meta‐analysis (Figures S7 and S8).
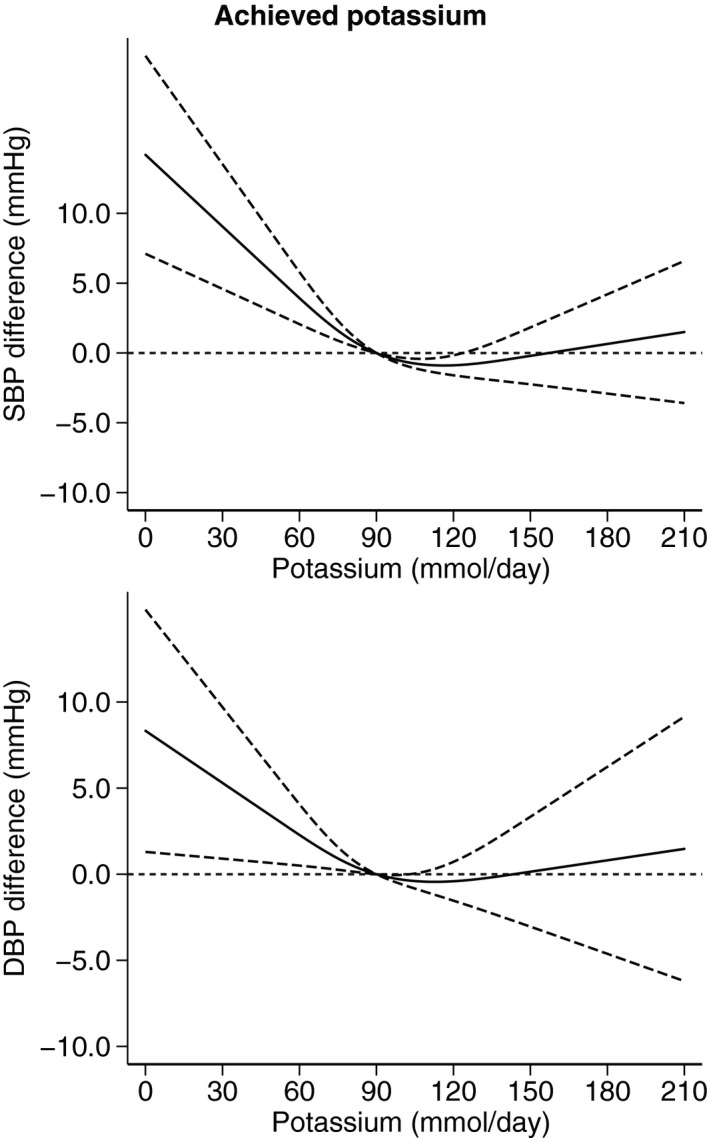
As reported in Figure 4, dose‐response analysis according to hypertension status, after removing trials performed in “mixed” samples with normal and high BP, showed a small decrease in mean BP levels associated with an increased potassium excretion up to 20 to 30 mmol/d in both normotensive and hypertensive trials, although in the latter the hypotensive effect of potassium was larger and occurred within a larger range of higher potassium excretion in supplemented participants (up to 90 mmol/d, versus a threshold of 60 mmol/d in those with no hypertension). For the increased BP levels following high amounts of potassium supplementation in participants with hypertension (Figure 5), it was considerably more evident in those receiving pharmacological treatment (starting at ≈60 mmol/d of difference in potassium excretion for the supplemented participants) compared with their counterparts not taking medications, for whom the BP increase started to occur at ≈110 mmol/d of excess potassium excretion. Further investigations to explore the effect of different antihypertensive treatment could not be performed because the original data did not report such stratified analyses by type of medication.
Figure 4. Dose‐response meta‐analysis of changes in systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels (as mm Hg), according to differences in potassium excretion between the treatment arms at the end of the trials in participants with no hypertension (N=5) and with hypertension (N=27).
Spline curve (solid line) with 95% confidence limits (long dashed lines).
Figure 5. Dose‐response meta‐analysis of changes in systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels (as mm Hg), according to differences in potassium excretion between the treatment arms at the end of the trials in subjects with hypertension not taking antihypertensive medications (N=22) and using antihypertensive medications (N=6).
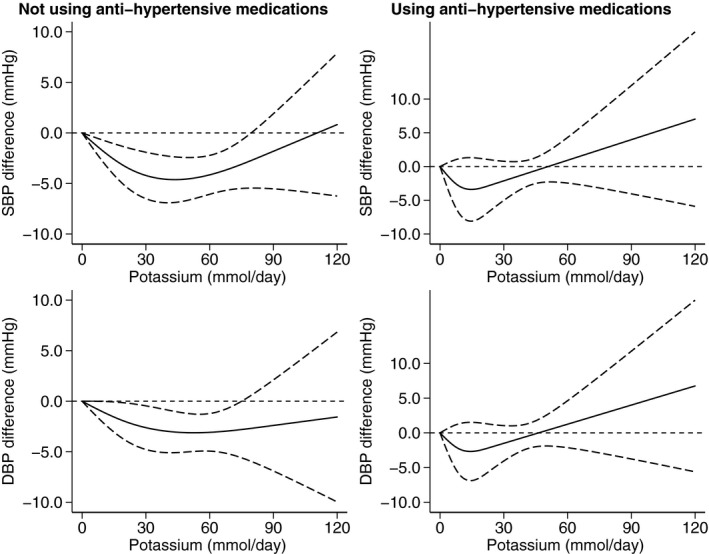
Spline curve (solid line) with 95% confidence limits (long dashed lines).
When we performed a conventional forest plot analysis, it showed a larger BP decrease following potassium supplementation in those with compared with those without hypertension (Figure S9). Among those with hypertension, potassium supplementation was on average more effective in lowering BP in participants not using antihypertensive medication compared with those receiving antihypertensive drug treatment (Figure S10). Considering the effects of achieved potassium excretion according to hypertension status and using 90 mmol/d as the reference value (Figure 6), in those in the normal BP category, we observed increasing BP levels for decreasing potassium exposure below the reference value, whereas >90 mmol/d DBP slightly increased while this did not occur for SBP. In participants with hypertension, the range of 90 to 120 mmol/d was associated with the lowest BP values, whereas above and much more strongly below this range, both SBP and DBP increased. In this subgroup, taking or not antihypertensive drugs did not appear to be associated with major changes in the effect of achieved potassium intake on BP levels (Figure 7).
Figure 6. Dose‐response meta‐analysis of changes in systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels (as mm Hg), according to achieved potassium excretion levels between arms at the end of the trials in participants with no hypertension (N=5) and with hypertension (N=27).
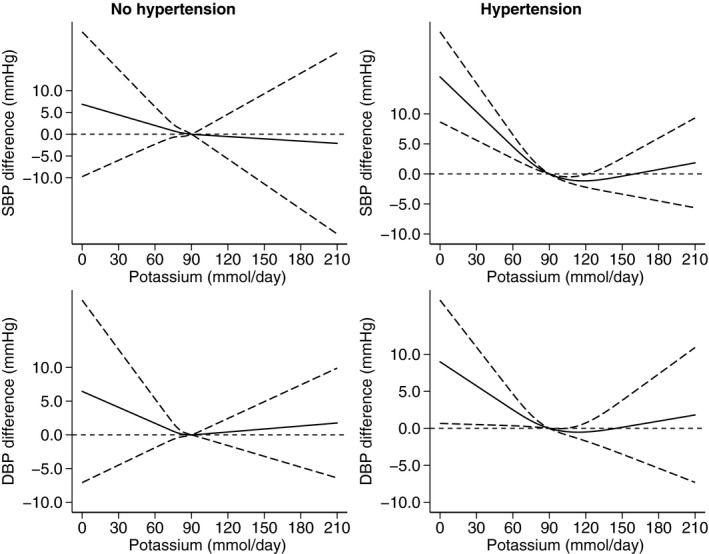
Spline curve (solid line) with 95% confidence limits (long dashed lines).
Figure 7. Dose‐response meta‐analysis of changes in systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels (as mm Hg), according to achieved potassium excretion levels between arms at the end of the trials in participants with hypertension not taking antihypertensive medications (N=22) and using antihypertensive medications (N=6).
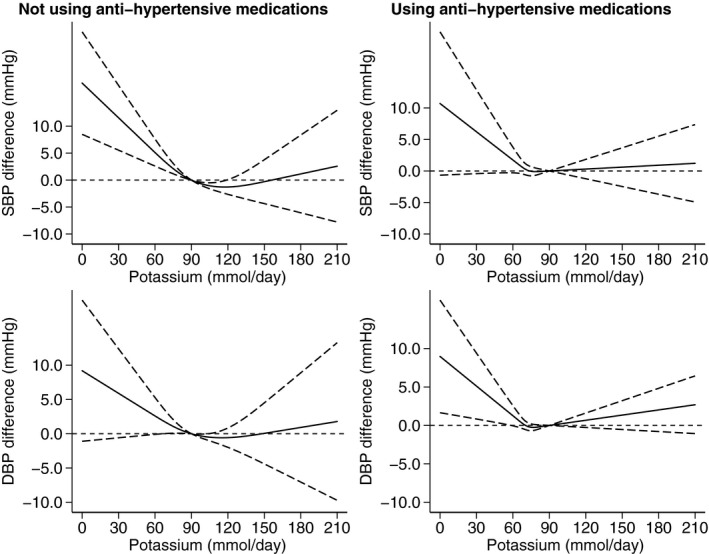
Spline curve (solid line) with 95% confidence limits (long dashed lines).
In an analysis stratified by trial design (crossover versus parallel), the dose‐response analysis showed a larger BP decrease in the latter group, but there was a higher increase in BP in those receiving the largest supplementation, starting at approximately a higher excretion of ≈30 mmol/d, in either the overall population or those with hypertension (Figures S11 and S12). The corresponding forest plot analysis showed a larger BP decrease in the crossover studies, in the total sample and analyses restricted to those with hypertension (Figures S13 and S14).
In a dose‐response analysis based on pretreatment potassium excretion, a larger effect on mean BP difference was noted in the studies with a urinary potassium <75 mmol/d (Figure S15). Corresponding forest plot analyses showed a consistent pattern of a slightly higher BP‐lowering effect (Figure S16).
Dose‐response analyses stratified by increasing level of baseline sodium excretion showed that potassium supplementation had different effects on BP values, according to level of sodium excretion (Figure 8), as depicted in the forest plot analysis (Figure S17). Both the lowering and the enhancing effects on BP induced by potassium supplementation were much weaker in the bottom category of sodium intake, <3000 mg/d, particularly for DBP, whereas in the intermediate category of sodium exposure, the threshold from shifting from a BP‐lowering effect into a BP‐enhancing effect was ≈80 mmol/d of supplemental potassium excretion for SBP and 60 mmol/d for DBP. The highest category of sodium exposure showed the largest decrease of both SBP and DPB, with no evidence of any BP increase, even for the highest amount of potassium supplementation.
Figure 8. Dose‐response meta‐analysis of changes in systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels (as mm Hg), according to studies with baseline sodium (uNa) <3 g/d (N=8), between 3 and 4 g/d (N=17), and ≥4 g/d (N=9).
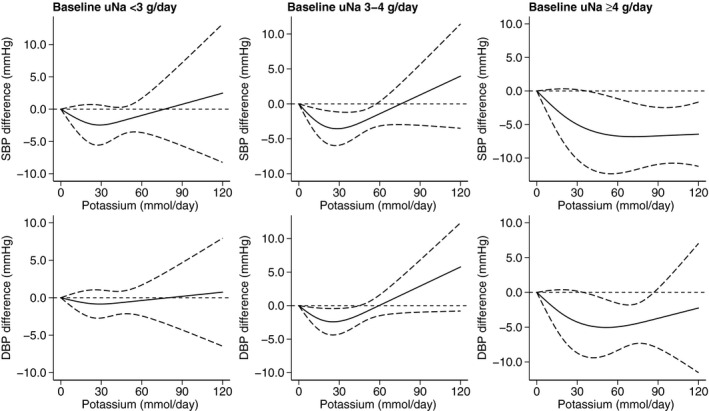
Spline regression curve (solid line) with 95% confidence limits (long dashed lines).
The modalities of BP measurement associated with the largest decreases were when BP was measured in the supine and standing positions, and when a manual device was used (Figures S18 through S21).
Analyses restricted to trials with a duration of ≥12 weeks (N=5) are shown in Figure S22. The analysis based on the amount of supplemental potassium showed a comparable trend to that observed in the entire set of studies, although there was evidence of an increased BP‐enhancing effect at a lower level of excess potassium exposure (ie, for <60 mmol/d of potassium difference between intervention and control arms), whereas this occurred at >60 mmol/d in the entire data set (Figure 2). For the analysis based on achieved potassium excretion at the end of the trial, the results of this subgroup analysis based on the longest duration studies showed that 90 mmol/d of potassium excretion was the amount associated with the most favorable effects on both SBP and DPB, with slightly lower estimates compared with the entire set of studies (Figure 3). However, in this subset of studies, there was no indication of an effect of high potassium intake in increasing DBP, which was different from what was observed in the entire set of studies. However, the effect estimates yielded by these analyses were statistically imprecise, because of the considerably lower number of studies compared with the overall trials available.
Funnel plots provided slight evidence of an asymmetric distribution for SBP (Figure S23), suggesting the possible occurrence of some publication bias. However, no such evidence emerged for DBP, thus reducing the likelihood of a major publication bias.
Discussion
The end point most investigated in studies assessing the relationship between potassium exposure and human health is BP. This is also the only end point for which a large number of experimental human studies are available, generally in the form of RCTs with either a crossover or a parallel design, this being the study design with the strongest level of evidence with reference to the risk of exposure misclassification and confounding.
Despite apparently strong evidence that potassium supplementation decreases SBP and DBP,12, 14, 18, 71 the exact dose‐response of the association has not been well established.13 The main reason for this is lack of a valid method for assessing dose‐response in the commonly used 2‐arm trial design that compares participants assigned to potassium supplementation or placebo. The biostatistical tools previously available for randomized comparison of dose‐response effect required at least 3 levels of exposure within each trial (independent of trial design), to allow calculation of a flexible, nonlinear dose‐response relationship between the exposure of interest and the outcome.19 This limitation has substantially hampered the use of human experimental studies for the accurate risk assessment of potassium supplementation,13 in both the general population and selected subgroups, such as those with or at high risk for hypertension. Attempts have been made to assess the dose‐response relationship between potassium intake and BP with meta‐regression models based on the assumption of a “straight‐line relationship”18 or forest plots based on comparison of the highest versus lowest intake levels, which in addition compare heterogeneous exposure categories.14 Unfortunately, none of these approaches allows detection and assessment of nonlinear dose‐response relationships.
By using a new “1‐stage” model that allows for inclusion of trials with only 2 levels of exposure, as is the case for most RCTs, we detected a dose‐response curve for the BP effects of potassium that was curvilinear across a wide range of treatment differences and absolute values of potassium exposure. This may have major implications in the risk assessment of potassium supplementation. Our finding of a U‐shaped relationship between potassium intake and BP was somewhat unexpected on the basis of previous clinical trial meta‐analyses and assessments. Although it confirms previous reports that a minimum dose of potassium is necessary for a BP‐lowering effect of potassium supplementation, it also suggests that high doses of potassium may result in a higher level of BP. The BP‐increasing effect of high potassium exposure was observed in both our overall results and the subgroup analyses of participants with hypertension or a normal level of BP, although being stronger in the former group. The optimal levels of “supplemental” (net difference between the 2 arms) and overall (achieved) potassium excretion appeared to be 30 and 90 to 130 mmol/d (1200 and 3500–5100 mg/d), respectively. The corresponding intakes would be higher (ie, by using the generally adopted conversion factor of 1.3,20, 21, 72, 73, 74, 75 ≈1500 mg/d of supplemental potassium and an overall intake of 4500–6500 mg/d). However, these estimates are based on a heterogeneous population mainly composed by adults with hypertension, and therefore not necessarily representing the general population. In addition, the estimates are based on experience in trials that disproportionally represent short‐term interventions. Estimates for those with a normal level of BP are lower than the aforementioned ones (ie, ≈800 mg/d of supplemental potassium and 4500 mg/d of total potassium intake), and these figures are consistent with those yielded by the trials of longer duration.
On the basis of the most recent observational epidemiologic literature, a tendency toward a U‐shaped effect of potassium supplementation on BP was not entirely unexpected. In a recent dose‐response meta‐analysis of nonexperimental epidemiologic studies, potassium intake appeared to have a dual relationship with the risk of stroke, lower at up to an intake of ≈90 mmol (3500 mg)/d, and higher at high levels.21 This pattern was noted both in BP adjusted and unadjusted analyses. In a Chinese community cohort study, participants with the lowest and highest intake of potassium had an increased risk of hypertension, although the increase was much higher in the latter group,22 thus suggesting that both rather low and high intakes of potassium may adversely affect BP levels. In the FHS (Framingham Heart Study), those with a higher level of serum potassium progressed to a higher level of BP or directly to hypertension during a 4‐year period of follow‐up,74 with a J‐shaped association for women and a U‐shaped association for men. However, participants with a potassium level >6.3 mmol were excluded, and the authors dismissed their results as being not “statistically significant.” In the BRHS (British Regional Heart Study), baseline potassium levels were positively associated with excess mortality, including increased CVD mortality.76 Results from the National Health and Nutrition Examination Survey I also showed higher CVD mortality for participants in the highest category of baseline serum potassium compared with the intermediate one, with the lowest exposure category also showing a (slightly) increased risk of death.77 In addition, the possibility that chronic hyperkalemia, usually defined on the basis of the general population distribution, has a U‐shaped association with general mortality is now being acknowledged24, 25 and has been a source of some concern, on the basis of the consistent results of several cohort studies performed in diseased, high‐risk or healthy participants.28, 78, 79, 80, 81, 82
The public health implications of our findings of a U‐shaped relationship between potassium excretion and BP levels appears to be considerably more important for a potassium intake that is too “low” rather than too “high,” also recognizing that the situation is different in clinical practice, where risk associated with hyperkaliemia has a different pattern11, 24 and therapy.83 In fact, potassium intake even in “acculturated” populations with an adequate diet tends to be lower, and sometimes much lower, than the adequate intake identified and recommended by risk assessment agencies, public health authorities, and professional societies.13, 20, 84 Therefore, dietary advice to increase potassium intake is likely to have beneficial effects and result in decreased BP levels in most populations. On the other hand, some populations and some selected subgroups and particularly some individuals (namely, those with hypertension treated with antihypertensive medication), if having a high baseline potassium intake, should be advised not to exceed the potassium intake levels found to be optimal in this meta‐analysis. This may also be true for individuals with low‐to‐intermediate sodium intake, because our analysis also suggests that those with a high sodium intake, as is typical in Western populations,85, 86, 87 benefit disproportionately from potassium supplementation and may also be more resistant to the BP increase following administration of a high potassium intake, suggesting an interaction between the 2 minerals. In addition, the number of studies was not enough to allow us to perform more detailed stratified subgroup analyses based on presence or absence of hypertension status and category of sodium intake, thus preventing us from verifying the presence of a possible interaction between hypertension status and sodium intake. Our BP estimates for the BP effect of a high potassium intake had wide CIs, making them less certain than BP effects at lower intakes of potassium, because of the small number of studies with relevant information at higher intakes of potassium and the resulting statistical imprecision of the effect estimate. In addition, the results based on achieved potassium excretion yielded little evidence of an increase in BP following a high potassium intake, further calling for caution about the effects of high intake of potassium on BP.
Our results also provide support for the population goals for potassium intake recently set by international authorities, such as the 90 mmol/d (3500 mg/d) adequate intake adopted by the European Food Safety Authority20 and the 87/66 mmol/d (3400–2600 mg/d) in men/women, recommended by the US National Academy of Medicine,13 based on the outcome of observational studies on potassium intake and several health end points, such as the risk of stroke for the adequate intake set by the European Food Safety Authority.
There is strong biological plausibility for a decrease in BP with a low intake of potassium, and some evidence to support an increase in BP at high levels of intake. Several experimental studies in laboratory settings and in animals have identified several mechanisms that may explain the BP‐lowering effect of potassium supplementation.88, 89, 90 Conversely, a high potassium intake could favor sodium excretion and an increase in renin activity and aldosterone levels, also dependent on preexisting electrolyte balance.11, 91, 92, 93, 94, 95
Limitations of our meta‐analysis and of the underlying studies include the fact that most of the trials included were of relatively short duration, including both the period of supplementation and follow‐up (median, 4 weeks). Despite exclusion of trials with <4 weeks of potassium supplementation and follow‐up, which may not reflect the long‐term effects of habitual potassium intake also attributable to the physiological adaptations that occur over time as a general response to dietary habits, extrapolation of our overall results to long‐term effects of potassium intake should still be made with caution. However, our analysis, restricted to the studies with the longest duration, yielded similar results and provides some reassurance that our findings may be extrapolated to longer periods of intake and therefore be more readily applicable to the general population. Also, our results, particularly in stratified analyses, were affected by statistical imprecision, particularly for the highest intakes of potassium and the longest duration of follow‐up, because of limited availability of studies in these settings.
In conclusion, this is the first meta‐analysis to investigate the effects of potassium supplementation on BP levels and with a specific focus on the dose‐response relationship. We found evidence for a nonlinear association, and for effect modification in those with hypertension, taking antihypertensive medication, or having a high sodium intake. Our findings for the effects of potassium intake on BP may explain, at least in part, the recently observed U‐shaped associations between serum potassium levels and risk of adverse outcomes in observational studies. They also support current European and US dietary recommendations for potassium intake and underscore the need to carefully address and manage potassium intake within comprehensive efforts to prevent CVD in both the general population and high‐risk subgroups.
Sources of Funding
This project was supported by grant GP‐EFSA‐AFSCO‐2017‐01 GA09 of the European Food Safety Authority (EFSA). The text reflects the authors’ views, and EFSA is not responsible for any use that may be made of the information it contains.
Disclosures
None.
Supporting information
Tables S1–S2 Figures S1–S23 References 38–70
(J Am Heart Assoc. 2020;9:e015719 DOI: 10.1161/JAHA.119.015719.)
For Sources of Funding and Disclosures, see page 14.
References
- 1. Key TJ, Appleby PN, Bradbury KE, Sweeting M, Wood A, Johansson I, Kuhn T, Steur M, Weiderpass E, Wennberg M, et al. Consumption of meat, fish, dairy products, and eggs and risk of ischemic heart disease. Circulation. 2019;139:2835–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Psaltopoulou T, Naska A, Orfanos P, Trichopoulos D, Mountokalakis T, Trichopoulou A. Olive oil, the Mediterranean diet, and arterial blood pressure: the Greek European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am J Clin Nutr. 2004;80:1012–1018. [DOI] [PubMed] [Google Scholar]
- 3. Gay HC, Rao SG, Vaccarino V, Ali MK. Effects of different dietary interventions on blood pressure: systematic review and meta‐analysis of randomized controlled trials. Hypertension. 2016;67:733–739. [DOI] [PubMed] [Google Scholar]
- 4. Flint AC, Conell C, Ren X, Banki NM, Chan SL, Rao VA, Melles RB, Bhatt DL. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. 2019;381:243–251. [DOI] [PubMed] [Google Scholar]
- 5. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e426–e483. [DOI] [PubMed] [Google Scholar]
- 6. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration . Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 7. Cook NR, Appel LJ, Whelton PK. Lower levels of sodium intake and reduced cardiovascular risk. Circulation. 2014;129:981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whelton PK. Body weight, sodium, potassium, and blood pressure. J Clin Hypertens (Greenwich). 2015;17:926–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gijsbers L, Molenberg FJ, Bakker SJ, Geleijnse JM. Potassium supplementation and heart rate: a meta‐analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2016;26:674–682. [DOI] [PubMed] [Google Scholar]
- 10. Whelton PK. Sodium and potassium intake in US adults. Circulation. 2018;137:247–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nomura N, Shoda W, Uchida S. Clinical importance of potassium intake and molecular mechanism of potassium regulation. Clin Exp Nephrol. 2019;23:1175–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Filippini T, Violi F, D'Amico R, Vinceti M. The effect of potassium supplementation on blood pressure in hypertensive subjects: a systematic review and meta‐analysis. Int J Cardiol. 2017;230:127–135. [DOI] [PubMed] [Google Scholar]
- 13. National Academy of Sciences (US) . The National Academies Collection: reports funded by National Institutes of Health In: Oria M, Harrison M, Stallings VA, eds. Dietary Reference Intakes for Sodium and Potassium. Washington, DC: National Academies Press (US); 2019:1–594. [PubMed] [Google Scholar]
- 14. Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta‐analyses. BMJ. 2013;346:f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, Klag MJ. Effects of oral potassium on blood pressure: meta‐analysis of randomized controlled clinical trials. JAMA. 1997;277:1624–1632. [DOI] [PubMed] [Google Scholar]
- 16. Geleijnse JM, Kok FJ, Grobbee DE. Blood pressure response to changes in sodium and potassium intake: a metaregression analysis of randomised trials. J Hum Hypertens. 2003;17:471–480. [DOI] [PubMed] [Google Scholar]
- 17. Iqbal S, Klammer N, Ekmekcioglu C. The effect of electrolytes on blood pressure: a brief summary of meta‐analyses. Nutrients. 2019;11:E1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Binia A, Jaeger J, Hu Y, Singh A, Zimmermann D. Daily potassium intake and sodium‐to‐potassium ratio in the reduction of blood pressure: a meta‐analysis of randomized controlled trials. J Hypertens. 2015;33:1509–1520. [DOI] [PubMed] [Google Scholar]
- 19. Newberry SJ, Chung M, Anderson CAM, Chen C, Fu Z, Tang A, Zhao N, Booth M, Marks J, Hollands S, et al. Sodium and Potassium Intake: Effects on Chronic Disease Outcomes and Risks. Comparative Effectiveness Review No. 206. Agency for Healthcare Research and Quality Publication No. 18‐EHC009‐EF. Rockville, MD; 2018. [PubMed] [Google Scholar]
- 20. EFSA Panel on Dietetic Products Nutrition and Allergies (NDA) , Turck D, Bresson J‐L, Burlingame B, Dean T, Fairweather‐Tait S, Heinonen M, Hirsch‐Ernst KI, Mangelsdorf I, McArdle H, Neuhäuser‐Berthold M, et al. Dietary reference values for potassium. EFSA J. 2016;14:e04592. [Google Scholar]
- 21. Vinceti M, Filippini T, Crippa A, de Sesmaisons A, Wise LA, Orsini N. Meta‐analysis of potassium intake and the risk of stroke. J Am Heart Assoc. 2016;5:e004210 DOI: 10.1161/JAHA.116.004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xi L, Hao YC, Liu J, Wang W, Wang M, Li GQ, Qi Y, Zhao F, Xie WX, Li Y, et al. Associations between serum potassium and sodium levels and risk of hypertension: a community‐based cohort study. J Geriatr Cardiol. 2015;12:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palaka E, Grandy S, Darlington O, McEwan P, van Doornewaard A. Associations between serum potassium and adverse clinical outcomes: a systematic literature review. Int J Clin Pract. 2020;74:e13421. [DOI] [PubMed] [Google Scholar]
- 24. Clase CM, Carrero JJ, Ellison DH, Grams ME, Hemmelgarn BR, Jardine MJ, Kovesdy CP, Kline GA, Lindner G, Obrador GT, et al. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020;97:42–61. [DOI] [PubMed] [Google Scholar]
- 25. Pitt B, Rossignol P. The association between serum potassium and mortality in patients with hypertension: “a wake‐up call.” Eur Heart J. 2017;38:113–115. [DOI] [PubMed] [Google Scholar]
- 26. Kovesdy CP, Appel LJ, Grams ME, Gutekunst L, McCullough PA, Palmer BF, Pitt B, Sica DA, Townsend RR. Potassium homeostasis in health and disease: a scientific workshop cosponsored by the National Kidney Foundation and the American Society of Hypertension. J Am Soc Hypertens. 2017;11:783–800. [DOI] [PubMed] [Google Scholar]
- 27. Kovesdy CP, Appel LJ, Grams ME, Gutekunst L, McCullough PA, Palmer BF, Pitt B, Sica DA, Townsend RR. Potassium homeostasis in health and disease: a scientific workshop cosponsored by the National Kidney Foundation and the American Society of Hypertension. Am J Kidney Dis. 2017;70:844–858. [DOI] [PubMed] [Google Scholar]
- 28. Kovesdy CP, Matsushita K, Sang Y, Brunskill NJ, Carrero JJ, Chodick G, Hasegawa T, Heerspink HL, Hirayama A, Landman GWD, et al. Serum potassium and adverse outcomes across the range of kidney function: a CKD Prognosis Consortium meta‐analysis. Eur Heart J. 2018;39:1535–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rodan AR. Potassium: friend or foe? Pediatr Nephrol. 2017;32:1109–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crippa A, Discacciati A, Bottai M, Spiegelman D, Orsini N. One‐stage dose‐response meta‐analysis for aggregated data. Stat Methods Med Res. 2019;28:1579–1596. [DOI] [PubMed] [Google Scholar]
- 31. Morgan RL, Whaley P, Thayer KA, Schunemann HJ. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. 2018;121:1027–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 33. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Filippini T, Hatch EE, Rothman KJ, Heck JE, Park AS, Crippa A, Orsini N, Vinceti M. Association between outdoor air pollution and childhood leukemia: a systematic review and dose‐response meta‐analysis. Environ Health Perspect. 2019;127:46002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Adani G, Filippini T, Wise LA, Halldorsson TI, Blaha L, Vinceti M. Dietary intake of acrylamide and risk of breast, endometrial, and ovarian cancers: a systematic review and dose‐response meta‐analysis. Cancer Epidemiol Biomarkers Prev. 2020; DOI: 10.1158/1055-9965. EPI‐19‐1628. [published online ahead of print, 2020 Mar 13]. [DOI] [PubMed] [Google Scholar]
- 36. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta‐analysis for linear and nonlinear dose‐response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Orsini N. DRMETA: Stata module for dose‐response meta‐analysis. Statistical Software Components S458546. Boston College Department of Economics, revised 25 May 2019. 2018. Available at: https://ideas.repec.org/c/boc/bocode/s458546.html. Accessed March 20, 2020. [Google Scholar]
- 38. Braschi A, Naismith DJ. The effect of a dietary supplement of potassium chloride or potassium citrate on blood pressure in predominantly normotensive volunteers. Br J Nutr. 2008;99:1284–1292. [DOI] [PubMed] [Google Scholar]
- 39. Berry SE, Mulla UZ, Chowienczyk PJ, Sanders TA. Increased potassium intake from fruit and vegetables or supplements does not lower blood pressure or improve vascular function in UK men and women with early hypertension: a randomised controlled trial. Br J Nutr. 2010;104:1839–1847. [DOI] [PubMed] [Google Scholar]
- 40. Barden AE, Vandongen R, Beilin LJ, Margetts B, Rogers P. Potassium supplementation does not lower blood pressure in normotensive women. J Hypertens. 1986;4:339–343. [DOI] [PubMed] [Google Scholar]
- 41. Bulpitt CJ, Ferrier G, Lewis PJ, Daymond M, Bulpitt PF, Dollery CT. Potassium supplementation fails to lower blood pressure in hypertensive patients receiving a potassium losing diuretic. Ann Clin Res. 1985;17:126–130. [PubMed] [Google Scholar]
- 42. Chalmers J, Morgan T, Doyle A, Dickson B, Hopper J, Mathews J, Matthews G, Moulds R, Myers J, Nowson C, et al. Australian National Health and Medical Research Council dietary salt study in mild hypertension. J Hypertens Suppl. 1986;4:S629–S637. [PubMed] [Google Scholar]
- 43. Forrester TE, Grell GA. Changes in red cell sodium content and blood pressure levels with potassium supplementation in black hypertensive patients. West Indian Med J. 1988;37:92–96. [PubMed] [Google Scholar]
- 44. Fotherby MD, Potter JF. Potassium supplementation reduces clinic and ambulatory blood pressure in elderly hypertensive patients. J Hypertens. 1992;10:1403–1408. [DOI] [PubMed] [Google Scholar]
- 45. Franzoni F, Santoro G, Carpi A, Da Prato F, Bartolomucci F, Femia FR, Prattichizzo F, Galetta F. Antihypertensive effect of oral potassium aspartate supplementation in mild to moderate arterial hypertension. Biomed Pharmacother. 2005;59:25–29. [DOI] [PubMed] [Google Scholar]
- 46. Gijsbers L, Dower JI, Mensink M, Siebelink E, Bakker SJ, Geleijnse JM. Effects of sodium and potassium supplementation on blood pressure and arterial stiffness: a fully controlled dietary intervention study. J Hum Hypertens. 2015;29:592–598. [DOI] [PubMed] [Google Scholar]
- 47. Graham UM, McCance DR, Young IS, Mullan KR. A randomised controlled trial evaluating the effect of potassium supplementation on vascular function and the renin‐angiotensin‐aldosterone system. J Hum Hypertens. 2014;28:333–339. [DOI] [PubMed] [Google Scholar]
- 48. Grimm RH, Kofron PM, Neaton JD, Svendsen KH, Elmer PJ, Holland L, Witte L, Clearman D, Prineas RJ. Effect of potassium supplementation combined with dietary sodium reduction on blood pressure in men taking antihypertensive medication. J Hypertens Suppl. 1988;6:S591–S593. [DOI] [PubMed] [Google Scholar]
- 49. Grobbee DE, Hofman A, Roelandt JT, Boomsma F, Schalekamp MA, Valkenburg HA. Sodium restriction and potassium supplementation in young people with mildly elevated blood pressure. J Hypertens. 1987;5:115–119. [DOI] [PubMed] [Google Scholar]
- 50. Gu D, He J, Wu X, Duan X, Whelton PK. Effect of potassium supplementation on blood pressure in Chinese: a randomized, placebo‐controlled trial. J Hypertens. 2001;19:1325–1331. [DOI] [PubMed] [Google Scholar]
- 51. Kaplan NM, Carnegie A, Raskin P, Heller JA, Simmons M. Potassium supplementation in hypertensive patients with diuretic‐induced hypokalemia. N Engl J Med. 1985;312:746–749. [DOI] [PubMed] [Google Scholar]
- 52. Kawano Y, Minami J, Takishita S, Omae T. Effects of potassium supplementation on office, home, and 24‐h blood pressure in patients with essential hypertension. Am J Hypertens. 1998;11:1141–1146. [DOI] [PubMed] [Google Scholar]
- 53. MacGregor GA, Markandu ND, Best FE, Elder DM, Cam JM, Sagnella GA, Squires M. Double‐blind randomised crossover trial of moderate sodium restriction in essential hypertension. Lancet. 1982;1:351–355. [DOI] [PubMed] [Google Scholar]
- 54. Matlou SM, Isles CG, Higgs A, Milne FJ, Murray GD, Schultz E, Starke IF. Potassium supplementation in blacks with mild to moderate essential hypertension. J Hypertens. 1986;4:61–64. [DOI] [PubMed] [Google Scholar]
- 55. Matthesen SK, Larsen T, Vase H, Lauridsen TG, Pedersen EB. Effect of potassium supplementation on renal tubular function, ambulatory blood pressure and pulse wave velocity in healthy humans. Scand J Clin Lab Invest. 2012;72:78–86. [DOI] [PubMed] [Google Scholar]
- 56. Miller JZ, Weinberger MH, Christian JC. Blood pressure response to potassium supplementation in normotensive adults and children. Hypertension. 1987;10:437–442. [DOI] [PubMed] [Google Scholar]
- 57. Overlack A, Stumpe KO, Moch B, Ollig A, Kleinmann R, Muller HM, Kolloch R, Kruck F. Hemodynamic, renal, and hormonal responses to changes in dietary potassium in normotensive and hypertensive man: long‐term antihypertensive effect of potassium supplementation in essential hypertension. Klin Wochenschr. 1985;63:352–360. [DOI] [PubMed] [Google Scholar]
- 58. Overlack A, Conrad H, Stumpe KO. The influence of oral potassium citrate/bicarbonate on blood pressure in essential hypertension during unrestricted salt intake. Klin Wochenschr. 1991;69(suppl 25):79–83. [PubMed] [Google Scholar]
- 59. Overlack A, Maus B, Ruppert M, Lennarz M, Kolloch R, Stumpe KO. Potassium citrate versus potassium chloride in essential hypertension: effects on hemodynamic, hormonal and metabolic parameters [in German]. Dtsch Med Wochenschr. 1995;120:631–635. [DOI] [PubMed] [Google Scholar]
- 60. Patki PS, Singh J, Gokhale SV, Bulakh PM, Shrotri DS, Patwardhan B. Efficacy of potassium and magnesium in essential hypertension: a double‐blind, placebo controlled, crossover study. BMJ. 1990;301:521–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Richards AM, Nicholls MG, Espiner EA, Ikram H, Maslowski AH, Hamilton EJ, Wells JE. Blood‐pressure response to moderate sodium restriction and to potassium supplementation in mild essential hypertension. Lancet. 1984;1:757–761. [DOI] [PubMed] [Google Scholar]
- 62. Siani A, Strazzullo P, Russo L, Guglielmi S, Iacoviello L, Ferrara LA, Mancini M. Controlled trial of long term oral potassium supplements in patients with mild hypertension. Br Med J (Clin Res Ed). 1987;294:1453–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Skrabal F, Gasser RW, Finkenstedt G, Rhomberg HP, Lochs A. Low‐sodium diet versus low‐sodium/high‐potassium diet for treatment of hypertension. Klin Wochenschr. 1984;62:124–128. [DOI] [PubMed] [Google Scholar]
- 64. Smith SJ, Markandu ND, Sagnella GA, MacGregor GA. Moderate potassium chloride supplementation in essential hypertension: is it additive to moderate sodium restriction? Br Med J (Clin Res Ed). 1985;290:110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sundar S, Sachdev KK, Vaish SK, Bhattacharya SK, Singh VP, Agarwal SK. Potassium supplementation in essential hypertension–a double blind placebo controlled study. J Assoc Physicians India. 1985;33:776–777. [PubMed] [Google Scholar]
- 66. Valdes G, Vio CP, Montero J, Avendano R. Potassium supplementation lowers blood pressure and increases urinary kallikrein in essential hypertensives. J Hum Hypertens. 1991;5:91–96. [PubMed] [Google Scholar]
- 67. Vongpatanasin W, Peri‐Okonny P, Velasco A, Arbique D, Wang Z, Ravikumar P, Adams‐Huet B, Moe OW, Pak CYC. Effects of potassium magnesium citrate supplementation on 24‐hour ambulatory blood pressure and oxidative stress marker in prehypertensive and hypertensive subjects. Am J Cardiol. 2016;118:849–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Whelton PK, Buring J, Borhani NO, Cohen JD, Cook N, Cutler JA, Kiley JE, Kuller LH, Satterfield S, Sacks FM, et al; Trials of Hypertension Prevention (TOHP) Collaborative Research Group. The effect of potassium supplementation in persons with a high‐normal blood pressure: results from phase I of the Trials of Hypertension Prevention (TOHP). Ann Epidemiol. 1995;5:85–95. [DOI] [PubMed] [Google Scholar]
- 69. TOHP Collaborative Research Group . The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels: results of the Trials of Hypertension Prevention, phase I. JAMA. 1992;267:1213–1220. [DOI] [PubMed] [Google Scholar]
- 70. He FJ, Marciniak M, Carney C, Markandu ND, Anand V, Fraser WD, Dalton RN, Kaski JC, MacGregor GA. Effects of potassium chloride and potassium bicarbonate on endothelial function, cardiovascular risk factors, and bone turnover in mild hypertensives. Hypertension. 2010;55:681–688. [DOI] [PubMed] [Google Scholar]
- 71. Whelton PK. Sodium, potassium, blood pressure, and cardiovascular disease in humans. Curr Hypertens Rep. 2014;16:465. [DOI] [PubMed] [Google Scholar]
- 72. Holbrook JT, Patterson KY, Bodner JE, Douglas LW, Veillon C, Kelsay JL, Mertz W, Smith JC Jr. Sodium and potassium intake and balance in adults consuming self‐selected diets. Am J Clin Nutr. 1984;40:786–793. [DOI] [PubMed] [Google Scholar]
- 73. Tasevska N, Runswick SA, Bingham SA. Urinary potassium is as reliable as urinary nitrogen for use as a recovery biomarker in dietary studies of free living individuals. J Nutr. 2006;136:1334–1340. [DOI] [PubMed] [Google Scholar]
- 74. WHO . Effect of increased potassium intake on cardiovascular disease, coronary heart disease and stroke. Geneva: World Health Organization; 2012. [Google Scholar]
- 75. Walsh CR, Larson MG, Vasan RS, Levy D. Serum potassium is not associated with blood pressure tracking in the Framingham Heart Study. Am J Hypertens. 2002;15:130–136. [DOI] [PubMed] [Google Scholar]
- 76. Wannamethee SG, Lever AF, Shaper AG, Whincup PH. Serum potassium, cigarette smoking, and mortality in middle‐aged men. Am J Epidemiol. 1997;145:598–606. [DOI] [PubMed] [Google Scholar]
- 77. Fang J, Madhavan S, Cohen H, Alderman MH. Serum potassium and cardiovascular mortality. J Gen Intern Med. 2000;15:885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Aldahl M, Jensen AC, Davidsen L, Eriksen MA, Moller Hansen S, Nielsen BJ, Krogager ML, Kober L, Torp‐Pedersen C, Sogaard P. Associations of serum potassium levels with mortality in chronic heart failure patients. Eur Heart J. 2017;38:2890–2896. [DOI] [PubMed] [Google Scholar]
- 79. Collins AJ, Pitt B, Reaven N, Funk S, McGaughey K, Wilson D, Bushinsky DA. Association of serum potassium with all‐cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gasparini A, Evans M, Barany P, Xu H, Jernberg T, Arnlov J, Lund LH, Carrero JJ. Plasma potassium ranges associated with mortality across stages of chronic kidney disease: the Stockholm CREAtinine Measurements (SCREAM) project. Nephrol Dial Transplant. 2019;34:1534–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Krogager ML, Torp‐Pedersen C, Mortensen RN, Kober L, Gislason G, Sogaard P, Aasbjerg K. Short‐term mortality risk of serum potassium levels in hypertension: a retrospective analysis of nationwide registry data. Eur Heart J. 2017;38:104–112. [DOI] [PubMed] [Google Scholar]
- 82. Matsushita K, Sang Y, Yang C, Ballew SH, Grams ME, Coresh J, Molnar MZ. Dyskalemia, its patterns, and prognosis among patients with incident heart failure: a nationwide study of US veterans. PLoS One. 2019;14:e0219899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tamargo J, Caballero R, Delpon E. New therapeutic approaches for the treatment of hyperkalemia in patients treated with renin‐angiotensin‐aldosterone system inhibitors. Cardiovasc Drugs Ther. 2018;32:99–119. [DOI] [PubMed] [Google Scholar]
- 84. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e563–e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, Ezzati M, Engell RE, Lim SS, Danaei G, Mozaffarian D, et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open. 2013;3:e003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Oliveira AC, Padrao P, Moreira A, Pinto M, Neto M, Santos T, Madureira J, Fernandes Ede O, Graca P, Breda J, et al. Potassium urinary excretion and dietary intake: a cross‐sectional analysis in 8‐10 year‐old children. BMC Pediatr. 2015;15:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cogswell ME, Loria CM, Terry AL, Zhao L, Wang CY, Chen TC, Wright JD, Pfeiffer CM, Merritt R, Moy CS, et al. Estimated 24‐hour urinary sodium and potassium excretion in US adults. JAMA. 2018;319:1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ellison DH, Terker AS. Why your mother was right: how potassium intake reduces blood pressure. Trans Am Clin Climatol Assoc. 2015;126:46–55. [PMC free article] [PubMed] [Google Scholar]
- 89. Gumz ML, Rabinowitz L, Wingo CS. An integrated view of potassium homeostasis. N Engl J Med. 2015;373:1787–1788. [DOI] [PubMed] [Google Scholar]
- 90. Tinker A, Aziz Q, Li Y, Specterman M. ATP‐sensitive potassium channels and their physiological and pathophysiological roles. Compr Physiol. 2018;8:1463–1511. [DOI] [PubMed] [Google Scholar]
- 91. Wu A, Wolley M, Stowasser M. The interplay of renal potassium and sodium handling in blood pressure regulation: critical role of the WNK‐SPAK‐NCC pathway. J Hum Hypertens. 2019;33:508–523. [DOI] [PubMed] [Google Scholar]
- 92. Vitzthum H, Seniuk A, Schulte LH, Muller ML, Hetz H, Ehmke H. Functional coupling of renal K+ and Na+ handling causes high blood pressure in Na+ replete mice. J Physiol. 2014;592:1139–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Castaneda‐Bueno M, Cervantes‐Perez LG, Rojas‐Vega L, Arroyo‐Garza I, Vazquez N, Moreno E, Gamba G. Modulation of NCC activity by low and high K(+) intake: insights into the signaling pathways involved. Am J Physiol Renal Physiol. 2014;306:F1507–F1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Poulsen SB, Fenton RA. K(+) and the renin‐angiotensin‐aldosterone system: new insights into their role in blood pressure control and hypertension treatment. J Physiol. 2019;597:4451–4464. [DOI] [PubMed] [Google Scholar]
- 95. Cheng CJ, Truong T, Baum M, Huang CL. Kidney‐specific WNK1 inhibits sodium reabsorption in the cortical thick ascending limb. Am J Physiol Renal Physiol. 2012;303:F667–F673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2 Figures S1–S23 References 38–70


