Figure 4: Analysis of metabolites in the plasma and urine of healthy rats after intravenous injection (IV) of 2-[18F]F-ENB.
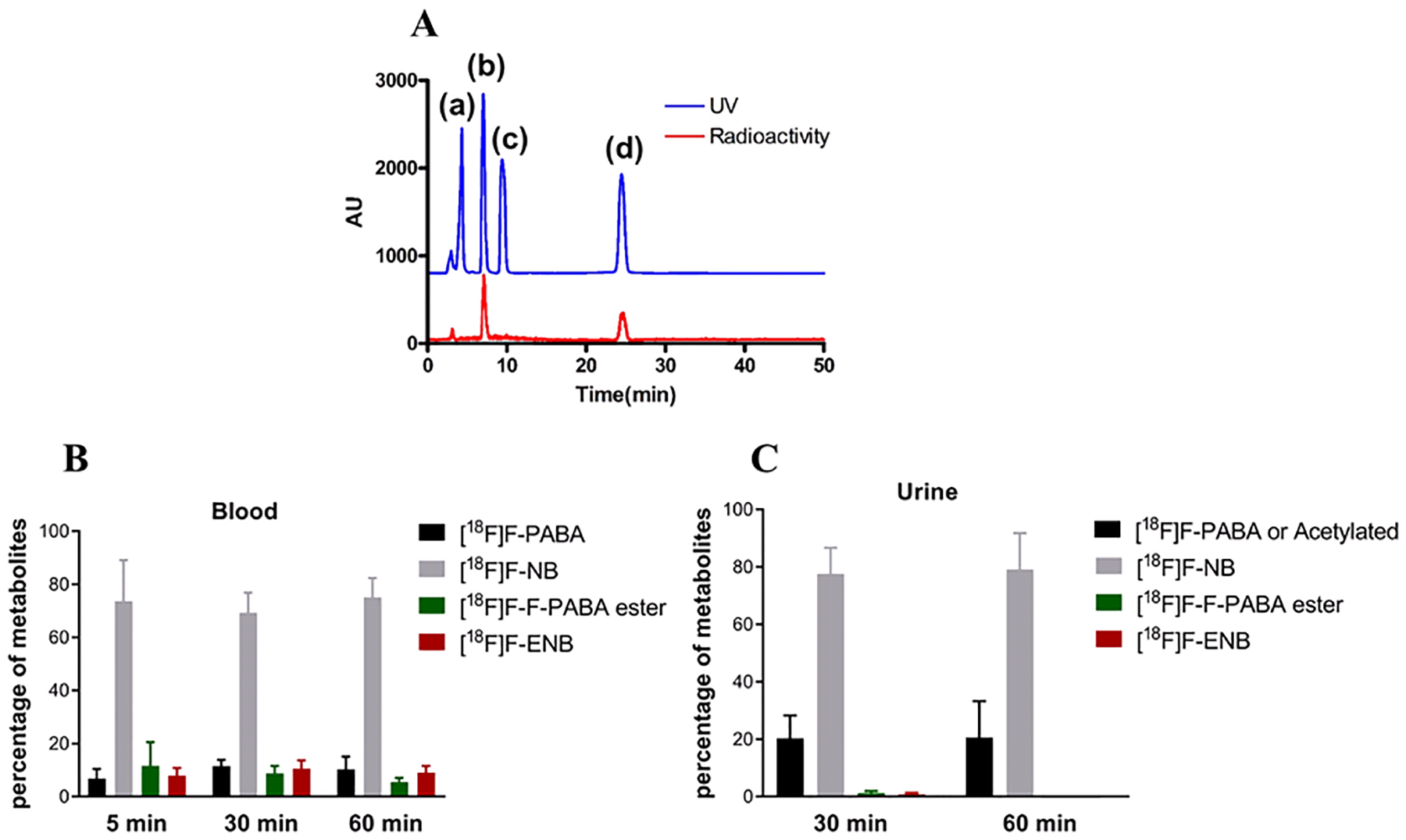
After IV administration of 2-[18F]F-ENB, the animals were sacrificed at each time point (n = 3 rats) and the blood and urine samples were collected for radio-HPLC analysis. (A) A representative HPLC chromatogram is shown to illustrate the separation of four major non-radioactive cold standards: 2-F-PABA (a), 2-F-NB (b), 2-F-PABA ester (c) and 2-F-ENB (d). The red trace is the HPLC chromatogram of a blood sample that reveals the presence of 2-[18F]F-NB and 2-[18F]F-ENB. The percent of each metabolite in blood (B) and urine (C) is shown.
