Abstract
Background:
Sevoflurane anesthesia induces Tau phosphorylation and cognitive impairment in neonatal but not in adult mice. Here we tested the hypothesis that differences in brain Tau amounts and in the activity of mitochondria-ATP-Nuak1-Tau cascade between the neonatal and adult mice contribute to the age-dependent effects of sevoflurane on cognitive function.
Methods:
6-day-old mice and 60-day-old mice from both sexes received anesthesia with 3% sevoflurane for two hours daily for three days. Biochemical methods were used to measure amounts of Tau, phosphorylated Tau, Nuak1, ATP concentrations and mitochondrial metabolism in the cerebral cortex and hippocampus. The Morris water maze test was used to evaluate cognitive function in the neonatal and adult mice.
Results:
Under baseline conditions and compared to 60-day-old mice, 6-day-old mice had higher amounts of Tau [2.6 ± 0.4 (arbitrary units, mean ± stand deviation) versus 1.3 ± 0.2, P < 0.001], Tau oligomer (0.3 ± 0.1 versus 0.1 ± 0.1, P = 0.008) and Nuak1 (0.9 ± 0.3 versus 0.3 ± 0.1, P = 0.025) while lesser amounts of ATP (0.8 ± 0.1 versus 1.5 ± 0.1, P < 0.001) and mitochondrial metabolism [74.8 ± 14.1 (pmoles/minute) versus 169.6 ± 15.3, P < 0.001] in the cerebral cortex. Compared to baseline conditions, sevoflurane anesthesia induced Tau phosphorylation at its serine 202/threonine 205 residues (1.1 ± 0.4 versus 0.2 ± 0.1, P < 0.001) in the 6-day-old, but not in the 60-day-old (0.05 ± 0.04 versus 0.03 ± 0.01, P = 0.186), mice. The sevoflurane-induced Tau phosphorylation and cognitive impairment in the neonatal mice were both attenuated by the inhibition of Nuak1 and the treatment of vitamin K2.
Conclusion:
Higher brain Tau concentrations and lower brain mitochondrial metabolism in neonatal compared to adult mice contribute to developmental stage-dependent cognitive dysfunction following sevoflurane anesthesia.
Keywords: anesthesia, age, Tau, Tau phosphorylation, Nuak1, ATP, mitochondria, cognition
Introduction
Young children who have had multiple exposures to anesthesia and surgery may experience a greater risk of developing neurocognitive disorder, although a single exposure to anesthesia and surgery may not be associated with such disorder.1,2 A recent prospective study revealed that children with multiple exposures to anesthesia/surgery do not have significant reductions in their intelligence quotients but develop impairments in processing speeds and fine motor abilities.3 However, other findings show that even multiple exposures of anesthesia and surgery do not cause cognitive dysfunction in children.4,5
In pre-clinical studies, anesthetics have been reported to induce cognitive impairment, apoptosis, synaptic deficiency, neuroinflammation, Tau phosphorylation, and other damage in young animals.2 These neurotoxic and cognitive impairment effects may also be mediated through endocrine abnormalities,6–11 histone acetylation,12 and epigenetic mechanisms.13 Our previous studies show that sevoflurane anesthesia induces Tau phosphorylation and cognitive impairment in 6-day-old neonatal mice but not 60-day-old adult mice.14–17 However, the underlying mechanism of these age-dependent effects remains unknown.
Tau is a neuronal protein that assembles and stabilizes microtubules.18 Tauopathy, the pathological aggregation of Tau protein in neurofibrillary or gliofibrillary tangles in the brain, is a hallmark of Alzheimer’s disease neuropathogenesis.19 Nuak1 is an AMPK-related kinase20 that can phosphorylate Tau protein at serine 356, which decreases Tau degradation, leading to accumulation of total Tau.21 However, the role of Tau in developmental anesthesia neurotoxicity and cognitive impairment in neonatal mice remains largely unknown. Specifically, whether Tau contributes to the difference in cognitive function between neonatal and adult mice following general anesthesia remains to be investigated.
The objective of the present study is to gain mechanistic insights into why sevoflurane anesthesia induces Tau phosphorylation and cognitive impairment in neonatal but not in adult mice. We hypothesized that differences in Tau amounts and Tau phosphorylation in brain tissues between neonatal and adult mice contribute to age-dependent differences in cognitive function following administration of sevoflurane anesthesia. Therefore, we compared amounts of Tau and phosphorylated Tau in the cortex and hippocampus of neonatal and adult mice from both sexes. We also compared amounts of Nuak1 as well as ATP concentrations and mitochondrial metabolism in brain tissues of these mice. We used a specific Nuak1 inhibitor, HTH-01–015,22 as well as vitamin K2, a mitochondrial electron carrier energy enhancer23 with neuroprotective effects,23–25 to further determine the extent to which variations in brain mitochondria activity, ATP concentrations, and amounts of Nuak1 and Tau between neonatal and adult mice contribute to differences in cognitive function following sevoflurane anesthesia.
Materials and Methods
Mice, anesthesia, and treatment.
The animal protocol was approved by the Standing Committee on Animals at Massachusetts General Hospital, Boston, MA (protocol 2006N000219). Efforts were made to minimize the number of animals used in the studies. The manuscript was written according to ARRIVE guidelines.
Adult mice (C57BL/6J) were purchased from the Jackson lab (Jackson Lab, Bar Harbor, ME). Neonatal mice from both sexes at postnatal day 6 were obtained from our own breeding. We anesthetized mice from both sexes with 3% sevoflurane for 2 hours at postnatal days 6, 7 and 8 based on our previous data showing that this treatment causes neuroinflammation, Tau phosphorylation, and cognitive impairment in neonatal mice.14,15 Given it is difficult to identify the sex of 6-day-old mice with certainty, and the objective of the present studies was not to determine sex-dependent effects, we did not allocate equal numbers of neonatal female or male mice in each group. Rather, neonatal mice from both sexes were used in the studies.
Importantly, during revision of this manuscript, we were asked to include adult male mice in additional studies to determine whether age-related differences existed between the neonatal mice from both sexes and the adult female mice also could appear between the neonatal mice from both sexes and the adult male mice. Therefore, at the completion of the present studies, we used the mixture of female and male 6-day-old mice (6-day-old mice from both sexes), and either female or male 60-day-old mice to determine the underlying mechanism by which the sevoflurane anesthesia only induced cognitive impairment in neonatal mice from both sexes. We called these 6-day-old mice from both sexes as the neonatal mice from both sexes or postnatal day 6 (female plus male) in the manuscript and figures, respectively. Similarly, we called the 60-day-old mice as adult mice or postnatal day 60 (female, male or female plus male) in the manuscript and figures, respectively. These mice were randomly assigned to the following groups: (1) baseline; (2) baseline plus DMSO (vehicle of HTH-01–015); (3) baseline plus HTH-01–015; (4) baseline plus corn oil (vehicle of vitamin K2); (5) baseline plus vitamin K2; (6) sevoflurane; (7) sevoflurane plus DMSO; (8) sevoflurane plus HTH-01–015; (9) sevoflurane plus corn oil; or (10) sevoflurane plus vitamin K2. Based on the results (e.g., immunohistochemistry, ELISA studies) and power analysis of the data (e.g., western blot, behavioral studies) from previous studies,14 we included ≥10 mice in each group for behavioral studies; 6 mice in each group for western blots, PCR, and ATP assays; 4 mice in each group for ELISA and mass spectrometry studies; and 3 mice in each group for immunostaining studies. Note that the additional experiments during the revision phases included adult male mice, thus we used different groups of neonatal mice from both sexes and adult male mice in these additional experiments. These different groups of neonatal mice from both sexes and male adult mice were also randomly assigned into the baseline and sevoflurane anesthesia groups. We used the same time of separation from mother as performed in our previous studies.14,15
Mice were anesthetized on postnatal days 6, 7, and 8 or on postnatal days 60, 61, and 62, as described in our previous studies,14 with 3% sevoflurane plus 60% oxygen for 2 hours daily for three days. Mice in baseline group only received 60% oxygen at an identical flow rate in similar chambers. We continuously monitored concentrations of sevoflurane and oxygen using a gas analyzer (Dash 4000; GE Healthcare, Milwaukee, WI) during anesthesia administration. Anesthesia chamber temperature was monitored and controlled by a feedback-based system with a DC temperature control system (World Precision Instruments Inc, Sarasota, FL, USA), which controls and automatically adjusts temperature to keep mouse rectal temperature at 37°C (±0.5°C) via a warming pad placed under this chamber. Anesthesia with 3% sevoflurane for two hours did not cause significant changes in skin and core temperature (Supplemental Figure 1a and b) or blood values of pH, pO2, and pCO2 as compared to the baseline (Supplemental Figure 1c–e).
In the intervention studies, we treated mice with HTH-01–015 (10 mg/kg, dissolved in DMSO at 0.45 μg/μL; Tocris Bioscience, UK)22 or vitamin K2 (100 mg/kg, dissolved in corn oil at 4.5 μg/μL; Sigma-Aldrich, St. Louis, MO)25 through intraperitoneal administration 30 minutes before each of the three treatments of sevoflurane anesthesia on postnatal days 6, 7 and 8 mice. The dosage of HTH-01–105 (10 mg/kg) was chosen based on our own observation that 10 mg/kg HTH-01–105 was able to decrease the amounts of Tau-PS356 in the brain tissues of mice. Mice in the vehicle treatment group received 0.1 mL vehicle (15 μL corn oil or DMSO dissolved in 1 mL saline).
Finally, we compared the Tau amounts between postnatal day 60 female and postnatal day 60 male mice, and between postnatal day 6 mice from both sexes and postnatal day 60 female or male mice to assess whether there were sex-dependent changes in Tau amounts between the neonatal mice from both sexes and the adult mice.
Morris water maze.
Morris water maze tests were performed as described in our previous studies.14,15 Please see Supplemental Information.
Brain tissue harvest, lysis, and protein quantification.
Mice were decapitated at the end of sevoflurane anesthesia on postnatal day 8 or postnatal day 62, and cortex and hippocampus were harvested. Please see Supplemental Information.
Reverse transcriptase polymerase chain reaction (RT-PCR).
RNA was harvested and isolated from mouse cortex and hippocampus tissues. Real-time RT-PCR was performed using the QuantiTect SYBR green real-time PCR kit (Qiagen, Valencia, CA). Please see Supplemental Information.
Western blot.
Total Tau and Nuak1 amounts were detected with anti-Tau 46 antibody (Cat# T9450, 55 kDa, 1:2000, Sigma-Aldrich) and anti-Nuak1 antibody (Cat# ab37641, 74 kDa, 1:1000, Abcam, Cambridge, MA). Tau-PS356 antibody (Cat# ab75603, 55 kDa, 1:1000, Abcam) was used to determine amounts of Tau phosphorylated at serine 356. AT8 antibody (Tau-PS202/PT205, Cat# MN1020, 55 kDa, 1:500, Thermo Scientific, Waltham, MA) was used to detect amounts of Tau phosphorylated at its serine 202 and threonine 205 residues. T22 antibody (Cat# ABN454, 55 kDa, 1:1000, Millipore, Billerica, MA) was used to measure amounts of Tau oligomers. Finally, antibody used to detect nontargeted protein β-actin (42 kDa, 1:5000, Sigma, St. Louis, MO) served as a baseline for loading differences in total protein amount. Western blot quantification was performed as described by Xie et al.26
Enzyme-linked immunosorbent assay (ELISA).
We used the mouse total Tau immunoassay ELISA kit (Cat# MBS724062, MyBiosource, San Diego, CA) to determine total Tau amounts in the cortex and hippocampus of mice at postnatal day 8 or postnatal day 62. Please see Supplemental Information.
ATP measurement.
Concentration of ATP in the cortex of mice was determined with an ATP colorimetric/fluorometric assay kit per manufacturer’s protocol (Cat# ab83355, Abcam) and using methods described in our previous studies.27
Immunohistochemistry.
Mice were anesthetized with sevoflurane briefly (3% sevoflurane for 5 min) and perfused transcardially with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde in 0.1 M phosphate buffer at pH 7.4. Mouse brain tissues were removed, stored at 4°C in paraformaldehyde, and frozen. We used a Leica cryostat (Buffalo Grove, IL) at −18°C to cut 10-μm frozen sections from mouse brain hemispheres, specifically from the prefrontal cortex, for immunostaining. Sections were mounted onto slides (Fisher Scientific, Pittsburgh, PA) and fixed in acetone for two hours. Please see Supplemental Information.
Multiplexed quantitative mass spectrometry-based phosphoproteomics.
Specifically, phosphopeptides were labeled with TMT reagents as described previously.28,29 TMT-labeled phosphopeptides were identified using a MS2 spectra and quantified with MultiNotch (simultaneous precursor selection) MS3 method28,30 in a data dependent mode. Precursor ion selection for MS3 spectra was done based on low-resolution MS2 spectral data using the three most intense fragment ions.31 Peptides were quantified based on TMT reported ion intensities in the collected MS3 spectra, as reported previously.28,29 Phosphorylation site localization was assigned using Ascore.32 Phosphopeptide quantitative data were normalized in a two-step procedure. First, average intensity of each species (protein or phosphopeptide) was calculated and normalized to the median of all average intensities. Second, to account for any mixing errors, intensity of each species was normalized from the ratio of the median intensity for a given TMT channel to the median of all species intensities. Please see Supplemental Information.
Mitochondrial metabolism on Seahorse XFp Extracellular Flux Analyzer.
Mitochondria were isolated from the hippocampus of postnatal day 6 and postnatal day 60 mice from both sexes. Please see Supplemental Information.
Statistics.
Based on results (e.g., immunohistochemistry and ELISA) and power analysis (e.g., Western blot and behavioral studies) from data obtained from our previous studies,14 inclusion of 10 mice per group for behavioral studies; 6 mice per group for western blot, PCR, ATP, and mitochondrial metabolism assays; 4 mice per group for ELISA and mass spectrometry studies; and 3 mice per group for immunostaining studies would provide statistically significant results. Specifically, a sample size of 10 per group provides >90% power to detect a 1.4-fold difference using a two-sided t-test at the 0.05 alpha level, assuming standard deviation (SD) of 70%. A sample size of 4–6 per group provides >80% power to detect a 0.5-fold difference using two-sided t-test at the 0.05 alpha level, assuming SD of 20%.14 These assumed SDs were higher than what we saw in our data, therefore these power calculations are conservative. We present data from biochemistry studies and escape latency of Morris water maze as mean ± SD; and platform crossing numbers from the Morris water maze is presented as median and interquartile range. Notably, in experiments comparing neonatal and adult mice, we defined adult mice as the reference group and set values of variables in the reference group mice without anesthesia for comparison. In experiments to assess the effects of sevoflurane anesthesia, we defined non-anesthetized neonatal or adult mice as the baseline and set values of their variables for comparison. All data were quantified and expressed as arbitrary units (western blot and ELISA) or real numbers (number of positive cells, ATP, and mitochondrial metabolism) of the reference group or baseline group.
Interaction between time and group factors was determined by using two-way ANOVA with repeated measurements to analyze the difference in learning curves (based on escape latency) between mice in the baseline and sevoflurane anesthesia groups in the Morris water maze test. Student’s t-test with Bonferroni correction was used to compare the difference in escape latency between mice in the baseline and sevoflurane anesthesia groups during each day of the Morris water maze test. Mann-Whitney test was used to determine the difference between the baseline and sevoflurane anesthesia groups in terms of platform crossing number. There were no missing data for variables of the Morris water maze test (escape latency and platform crossing number) during data analysis. Kruskal-Wallis test was used to determine the difference in multiple comparisons when data did not pass a normality test (e.g., Tau ELISA studies in Figure 1). Student’s t-test was used to determine the difference in two-group comparison when data passed a normality test. Two-way ANOVA and Student’s t-test with Bonferroni correction were used to determine the effect of age (postnatal day 6 versus postnatal day 60), treatment (baseline versus sevoflurane anesthesia), and the interaction of age and treatment in terms of total amounts of Tau, Nuak1, Tau-PS356, AT8 (Tau-PS202/PT205), and T22. One-way ANOVA and individual Student’s t-test with Bonferroni correction were used to determine differences among groups in terms of total Tau, Nuak1, Tau-PS356, AT8 (Tau-PS202/PT205), and T22 amounts. Student’s t-test was used to compare oxygen consumption rate between mice in the baseline and experimental groups. Finally, data for baseline brain amounts of Tau, Nuak1, ATP concentration, and mitochondrial metabolism from female and male adult mice were pooled together and compared with those obtained from neonatal mice from both sexes by Student’s t-test. P < 0.05 was considered statistically significant, and significance testing was two-tailed. Note that adjusted P-values of the Bonferroni correction were calculated by dividing real P-values with the size in experiments, and adjusted P-values are reported. Statistical analysis was conducted using GraphPad Prism software (version 8.0) and SPSS statistic software (version 21.0).
Figure 1. Difference in brain Tau amount between neonatal and adult mice.
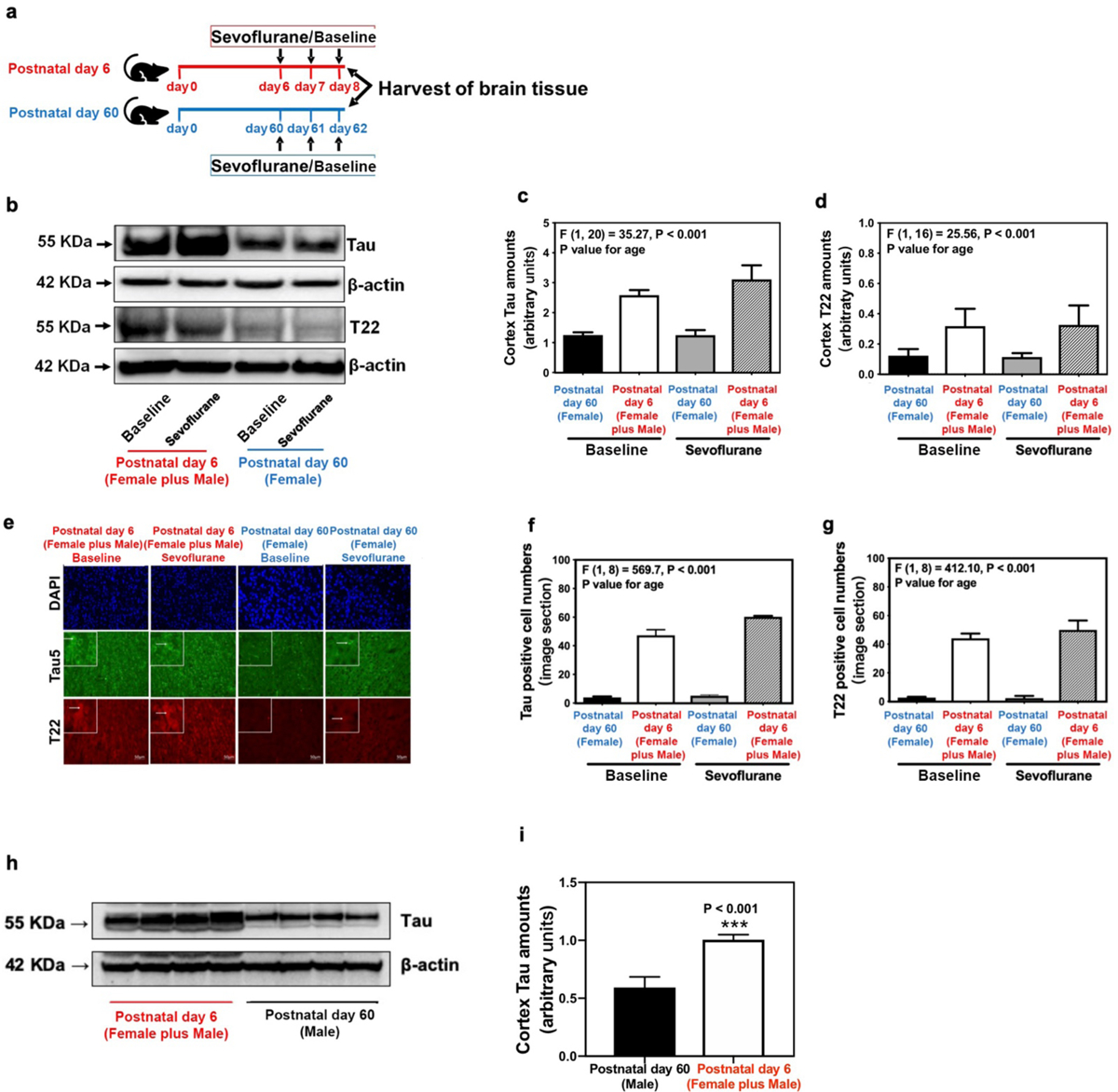
(a) Experimental design: Cortex or hippocampus tissues were harvested at the end of the baseline or sevoflurane anesthesia in neonatal (postnatal day 6 - postnatal day 8) and adult (postnatal day 60 - postnatal day 62) mice. (b) Difference in brain amounts of Tau and oligomer Tau (T22) in the cortex of postnatal day 6 mice from both sexes (neonatal mice from both sexes) and postnatal day 60 female mice [female reference group mice (Female)] in baseline and sevoflurane anesthesia groups. (c) Summary of amounts of Tau (n=6 mice/group). (d) Summary of amounts of oligomer Tau (T22) (n=6 mice/group). (e) Immunostaining of Tau- and T22-positive cells in the cortex of neonatal mice from both sexes and female reference group mice in baseline and sevoflurane anesthesia groups. (f) Quantification of Tau-positive cells (n=3 mice/group). (g) Quantification of T22-positive cells (n=3 mice/group). (h) Difference in brain amounts of Tau and oligomer Tau (T22) in the cortex of postnatal day 6 mice from both sexes (neonatal mice from both sexes) and postnatal day 60 male mice [male reference group mice (Male)] in baseline. (i) Summary of amounts of Tau (n=6 mice/group). Data are presented as mean ± standard deviation (SD). All data are quantified and expressed as arbitrary units or real numbers compared to reference group mice (postnatal day 60) or baseline group (non-anesthetized mice). PS, phosphorylated serine; PT, phosphorylated threonine. Two-way ANOVA: (c), (d), (f) and (g). Student’s t-test: (i). ***P<0.001.
Results
Neonatal mice have higher brain amounts of Tau and Tau oligomers than adult mice.
To evaluate whether the age-dependent neurocognitive responses to sevoflurane could be related to age-specific differences in brain amounts of Tau and phosphorylated Tau between neonatal and adult mice, we first compared the amounts of these proteins between 6-day-old and 60-day-old mice in baseline and following repeated exposures to sevoflurane (Figure 1a). Western blot analysis of protein expressions revealed that 6-day-old mice had greater amounts of Tau and Tau oligomers in the cortex than 60-day-old mice both in baseline [60-day-old female mice (reference group): 1.3 ± 0.2 arbitrary unit versus 6-day-old mice: 2.6 ± 0.4 arbitrary unit, P < 0.001], and after sevoflurane anesthesia [60-day-old female mice (reference group): 1.2 ± 0.4 arbitrary unit versus 6-day-old mice: 3.1 ± 1.2 arbitrary unit, P = 0.004] (Figure 1b–c). Tau oligomers were also comparably greater in the neonatal mice both in baseline [0.3 ± 0.1 (arbitrary unit) versus 0.1 ± 0.1 (arbitrary unit), P = 0.008) and following sevoflurane anesthesia [0.3 ± 0.2 (arbitrary unit) versus 0.1 ± 0.1 (arbitrary unit), P = 0.009] (Figure 1b and 1d). Immunohistochemical analysis (Figure 1e–g) and ELISA measurements (Supplementary Figure 2a–b) further confirmed the greater amounts of Tau and Tau oligomers in the cerebral cortex of neonatal mice compared to adult counterparts both in baseline and following sevoflurane exposures. Age-specific differences in Tau and Tau oligomer concentrations were also present in the hippocampus of the neonatal mice (Supplemental Figure 2c–e).
In our original work, we compared Tau amounts in neonatal mice from both sexes to adult female mice. During the peer review process, we performed additional experiments to exclude potential sex-specific effects. We found that adult male mice, like their female counterpart, also had lower brain Tau amounts than the neonatal mice from both sexes (1.0 ± 0.1 arbitrary unit versus 0.6 ± 0.1 arbitrary unit, P < 0.001) (Figure 1h–i), arguing against the potential sex-specific effect.
Repeated exposures to sevoflurane induce brain Tau phosphorylation in neonatal but not in adult mice.
Since Tau phosphorylation may impede the degradation of Tau, we next compared Tau phosphorylation between 6-day-old and 60-day-old mice both in baseline and following sevoflurane exposure. Western blot analysis revealed that 6-day-old mice had higher amounts of phosphorylated Tau at its serine 356 (Tau-PS356), serine 202 and threonine 205 (Tau-PS202/PT205) residues both in baseline and following repeated sevoflurane exposures when compared to 60-day-old female mice as a reference group (Figure 2a–c). Repeated exposure to sevoflurane increased the amounts of Tau-PS202/PT205 in the cerebral cortex of the neonatal (1.1 ± 0.4 arbitrary unit versus 0.2 ± 0.1 arbitrary unit, P < 0.001), but not of the adult (0.05 ± 0.04 versus 0.03 ± 0.01, P = 0.186), mice as compared to the baseline non-anesthetized conditions (Figure 2a–c). As in the cerebral cortex, Tau-PS365 and Tau-PS2020/PT205 were also higher in the hippocampus of neonatal mice compared to the female reference group (Supplemental Figure 2c–e). In line with these observations, multiplexed quantitative mass spectrometry-based phosphoproteomics29 showed more sites and higher amounts of pTau in the cortex of neonatal mice from both sexes compared to the female reference group mice (Supplemental Figure 3a–b). In an additional sets of experiments, per the peer review request to exclude potential sex-specific effects, we compared and found no difference in Tau-PS356 and Tau-PS202/PT205 amounts between the cerebral cortex of adult male and female mice (Supplemental Figure 4a–c).
Figure 2. Difference of brain phosphorylated Tau between neonatal and adult mice.
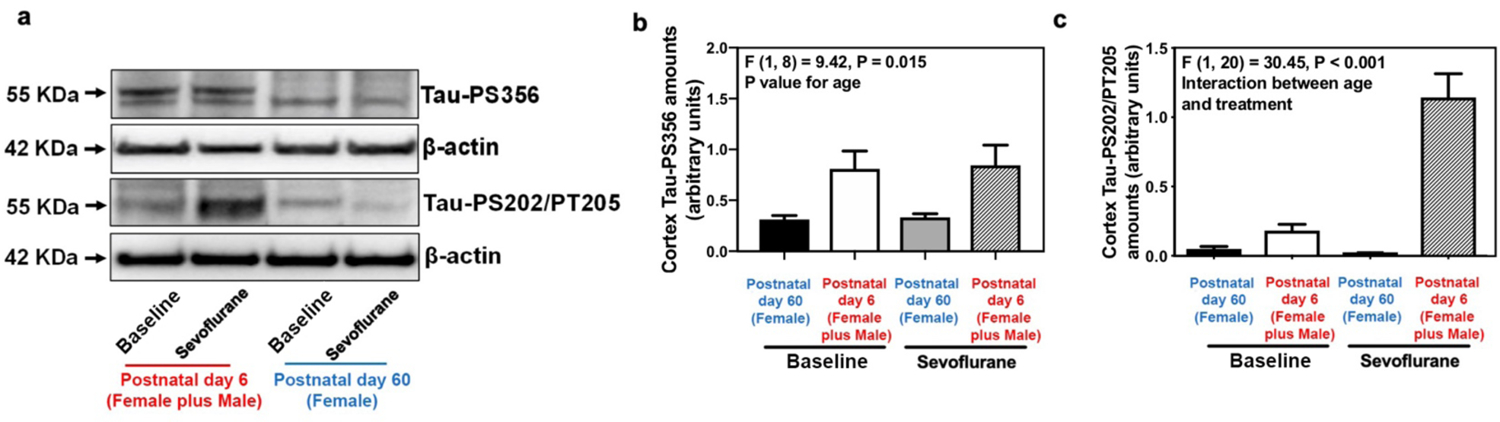
(a) Difference in brain amounts of Tau-PS356 and Tau-PS202/PT205 in the cortex of postnatal day 6 mice from both sexes (neonatal mice from both sexes) and postnatal day 60 female mice [female reference group mice (Female)] in baseline and sevoflurane anesthesia groups. Summary of amounts of Tau-PS356 (b) and Tau-PS202/PT205 (c) (n=6 mice/group). All data are quantified and expressed as arbitrary units compared to reference group mice (postnatal day 60) or baseline group (non-anesthetized mice). PS, phosphorylated serine; PT, phosphorylated threonine; Two-way ANOVA: (b) and (c).
Neonatal mice have higher brain concentrations of Nuak1 than adult mice
Nuak1, an AMPK-related kinase,20 phosphorylates Tau protein at serine 356, which then decreases degradation of Tau and leads to accumulation of total Tau.21 We therefore compared the amounts of Nuak1 in brain tissues between 6-day-old mice from both sexes and 60-day-old female mice (as a reference group) both in baseline non-anesthetized conditions and following repeated exposures to sevoflurane (Figure 3a). Neonatal mice from both sexes had lower amounts of phosphorylated Nuak1 in the cortex than the adult female (reference group) mice (Figure 3b). Moreover, neonatal mice from both sexes had higher amounts of Nuak1 in the cortex than the female reference group mice (F = 41.43, P < 0.001, two-way ANOVA and Student’s t-test with Bonferroni correction of 3 tests) in baseline (0.9 ± 0.3 arbitrary unit versus 0.3 ± 0.1 arbitrary unit, P = 0.025) or sevoflurane anesthesia (0.9 ± 0.1 arbitrary unit versus 0.3 ± 0.1 arbitrary unit, P < 0.001) groups (Figure 3c–d). Comparable findings were demonstrated in the hippocampus (Supplemental Figure 5a–b). In contrast, no significant difference was found in mRNA expression of Nuak1 in the cortex (Figure 3e) and hippocampus (Supplemental Figure 5c) between neonatal and adult mice. Per peer review request, we also compared Nuak1 concentrations in the cerebral cortex between neonatal mice from both sexes to adult male mice as a reference group and found comparable differences (60-day-old male mice: 0.5 ± 0.1 arbitrary unit versus 6-day-old mice from both sexes: 0.8 ± 0.1 arbitrary unit, P < 0.001) (Figure 3f–g).
Figure 3. Nuak1 and ATP concentrations in brains of neonatal and adult mice.
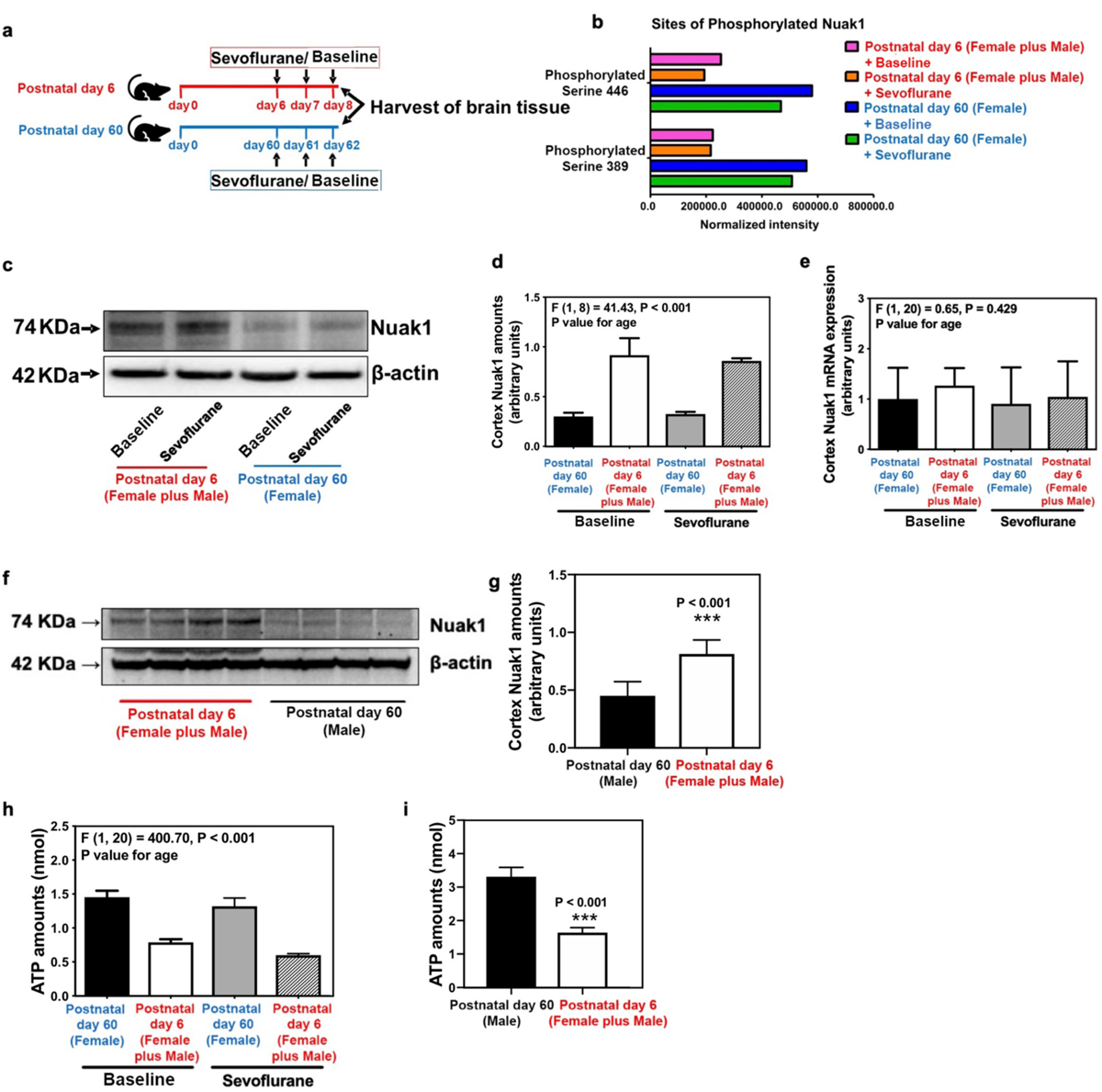
(a) Experimental design: Cortex and hippocampus were harvested at the end of the baseline or sevoflurane anesthesia in neonatal mice from both sexes and reference group mice. (b) Quantitative mass spectrometry data display amounts of phosphorylated Nuak1 in the cortex of neonatal mice from both sexes and female reference group mice in baseline and sevoflurane anesthesia groups. (c) Nuak1 protein amounts in the cortex of neonatal mice from both sexes and female reference group mice in baseline and sevoflurane anesthesia groups. (d) Summary of cortex Nuak1 protein amounts. (e) Difference in Nuak1 mRNA expression in the cortex of neonatal mice from both sexes and female reference group mice in baseline and sevoflurane anesthesia groups. (f) Nuak1 protein amounts in the cortex of neonatal mice from both sexes and male reference group mice. (g) Summary of Nuak1 protein amounts in the cortex of neonatal mice from both sexes and male reference group mice. (h) ATP concentrations in the cortex of neonatal mice from both sexes and female reference group mice in baseline and sevoflurane anesthesia groups (n=6 mice/group). (i) ATP concentrations in the cortex of neonatal mice from both sexes and male reference group mice (n=6 mice/group). Data are presented as mean ± standard deviation (SD). All data are quantified and expressed as arbitrary units or real numbers compared to reference group mice (postnatal day 60) or the baseline group (non-anesthetized mice). ATP, adenosine triphosphate. Two-way ANOVA: (d), (e), and (h). Student’s t-test: (g) and (i). ***P<0.001.
Since Nuak1 phosphorylation is an ATP-dependent process, we evaluated whether the less Nuak1 phoyphorylation observed in the neonatal mice is correlated with lower concentrations of ATP in brain tissues of neonatal versus adult mice. We found that the neonatal mice from both sexes exhibited lower baseline ATP concentrations in the cortex as compared to the female reference group mice (0.8 ± 0.1 arbitrary unit versus 1.5 ± 0.1 arbitrary unit, P < 0.001, Figure 3h) or male reference group mice (Figure 3i). There were no significant differences in cortex Nuak1 amounts (Supplemental Figure 6a–b) and ATP concentrations (Supplemental Figure 6c) between female and male postnatal day 60 mice either in the baseline non-anesthetized conditions or following sevoflurane exposure. Finally, sevoflurane anesthesia did not induce cognitive impairment in postnatal day 60 female (Supplemental Figure 6d) or male mice (Supplemental Figure 6e).
Nuak1 inhibitor HTH-01–015 inhibits the sevoflurane-induced Tau phosphorylation and cognitive impairment in neonatal mice
To further test the hypothesis that lower ATP concentrations reduce Nuak1 phosphorylation in brain tissues of neonatal mice, thereby leading to higher Nuak1 and Tau amounts, we treated 6-day-old mice from both sexes with a specific Nuak1 inhibitor HTH-01–01522 (Figure 4a). HTH-01–015 treatment did not affect brain ATP concentrations in the cerebral cortex of 6-day-old mice (Figure 4b). In contrast, Western blot analysis (Figure 4c–e) and ELISA (Figure 4f) revealed that HTH-01–015 treatment did not alter total Tau amounts but decreased Tau oligomer amounts in the cortex and hippocampus (Supplemental Figure 7a–e) of neonatal mice as compared to vehicle treatment.
Figure 4. Effects of Nuak1 inhibitor HTH-01–015 on Tau and oligomer Tau amounts in neonatal mice from both sexes.
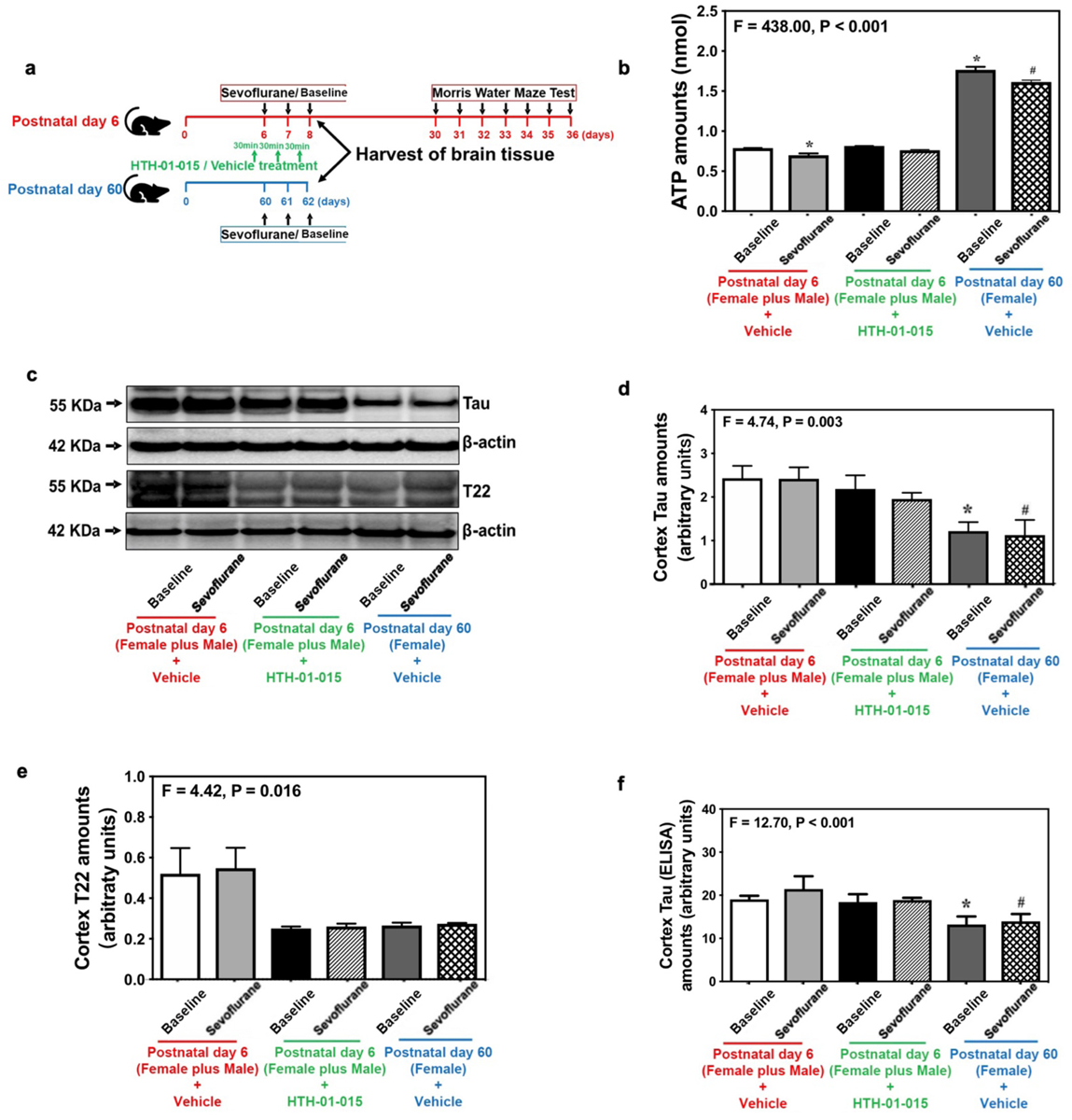
(a) Experimental design: Mice were treated with baseline or sevoflurane anesthesia with vehicle (DMSO) or HTH-01–015. (b) ATP concentration in cortex of neonatal mice from both sexes and female reference mice in baseline and sevoflurane anesthesia groups (n=6 mice/group). (c) Amounts of Tau and oligomer Tau (T22) in the cortex of neonatal mice from both sexes and female reference group mice. (d) Summary of Tau (n=6 mice/group). (e) Summary of Tau 22 (n=6 mice/group). (f) ELISA of total Tau in cortex of neonatal mice from both sexes and female reference group mice (n=6 mice/group). Data are presented as mean ± standard deviation (SD). All data are quantified and expressed as arbitrary units or real number compared to baseline group (non-anesthetized mice). PS, phosphorylated serine; PT, phosphorylated threonine; ELISA, enzyme-linked immunosorbent assay; DMSO, dimethylsulfoxide. One-way ANOVA: (b), (d), (e) and (f). *P<0.05; #P<0.05.
In line with these observations, we also found that HTH-01–015 decreased the amounts of both Tau-PS356 and Tau-PS202/PT205 as compared to vehicle treatment in either the baseline or sevoflurane anesthesia (Figure 5a–c). Comparable findings were obtained from hippocampal tissue (Supplemental Figure 7c–d).
Figure 5. Regulation of Tau phosphorylation and cognitive function by Nuak1 inhibitor HTH-01–015 in neonatal mice from both sexes.

(a) Amounts of Tau-PS356 and Tau-PS202/PT205 in the cortex of neonatal mice from both sexes and female reference group mice. (b) Summary of Tau-PS356 (n=6 mice/group). (c) Summary of Tau-PS202/PT205 (n=6 mice/group). Escape latency (d) and platform crossing number (e) of MWM in neonatal mice for baseline and sevoflurane anesthesia groups following pre-treatment with vehicle (n=10 mice/group). Escape latency (f) and platform crossing number (g) of MWM in neonatal mice for baseline and sevoflurane anesthesia groups following pre-treatment with HTH-01–015 (n=10 mice/group). Data are presented as mean ± standard deviation (SD). All data are quantified and expressed as arbitrary units or real numbers compared to baseline group (non-anesthetized mice). PS, phosphorylated serine; PT, phosphorylated threonine; ELISA, enzyme-linked immunosorbent assay; DMSO, dimethylsulfoxide. One-way ANOVA: (b) and (c). Two-way ANOVA with repeated measurement: (d) and (f). Mann-Whitney test: (e) and (g). *P<0.05; **P<0.01; ***P<0.001; #P<0.05.
In 6-day-old mice, sevoflurane anesthesia induced cognitive impairment as measured via Morris water maze tests from P30–P36 on escape latency (F=5.40, P<0.001, two-way ANOVA) and platform crossing number (P=0.004, Mann-Whitney test) (Figure 5d–e) as compared to baseline non-anesthetized condition. However, in mice pre-treated with HTH-10–105, sevoflurane anesthesia did not induce cognitive impairment, as evidenced by no significant interaction [F=0.71, P=0.642] between treatment and time on escape latency. Moreover, sevoflurane anesthesia did not decrease platform crossing number as compared to baseline in mice pre-treated with HTH-10–105 (P=0.541, Mann-Whitney test) (Figure 5f–g).
Vitamin K2 mitigates the sevoflurane anesthesia-induced Tau phosphorylation and cognitive impairment in neonatal mice
We next used vitamin K2, a mitochondrial electron carrier and energy enhancer23 with neuroprotective effects,23–25 to determine whether increasing concentration of ATP and the resulting decrease in Nuak1-dependent Tau phosphorylation could protect neonatal mice from sevoflurane exposure-associated cognitive impairment (Figure 6a). In contrast to Nuak1 inhibitor HTH-01–015, vitamin K2 increased brain ATP concentrations in neonatal mice from both sexes as compared to vehicle (F=101.40, P<0.001, one-way ANOVA and Student’s t-test with Bonferroni correction of 5 tests), which occurred in either the baseline (non-anesthetized, vitamin K2: 1.1 ± 0.1 arbitrary unit versus non-anesthetized, vehicle: 0.8 ± 0.1 arbitrary unit, P < 0.001) or sevoflurane anesthesia (sevoflurane, vitamin K2: 1.1 ± 0.1 arbitrary unit versus sevoflurane, vehicle: 0.7 ± 0.1 arbitrary unit, P < 0.001) groups (Figure 6b).
Figure 6. Effects of vitamin K2 on amounts of Tau and oligomer Tau in brain tissues of neonatal mice from both sexes.

(a) Experimental design. (b) ATP concentration in cortex of neonatal mice from both sexes and female reference group mice following vehicle in sevoflurane anesthesia and baseline groups (n=6 mice/group). (c) Amounts of Nuak1, Tau, and oligomer Tau (T22) in the cortex of neonatal mice from both sexes and female reference group mice. Summary of amounts of Nuak1 (d), Tau (e) and oligomer Tau (f) (n=6 mice/group). (g) ELISA of total Tau in the cortex of neonatal mice from both sexes and female reference group mice (n=4 mice/group). Data are presented as mean ± standard deviation (SD). All data are quantified and expressed as arbitrary units or real numbers compared to baseline group (non-anesthetized mice). ATP, adenosine triphosphate; ELISA, enzyme-linked immunosorbent assay; PS, phosphorylated serine; PT, phosphorylated threonine. One-way ANOVA: (b), (d), (e), and (g). Kruskal-Wallis test: (f). *P<0.05; #P<0.05.
Vitamin K2 decreased amounts of Nuak1 (Figure 6c–d) and oligomer Tau (Figure 6c and 6f), but not total Tau (Figure 6c and 6e), in the cortex and hippocampus (Supplemental Figure 8a–b) of neonatal mice from both sexes as compared to vehicle. ELISA showed that vitamin K2 did not affect the Tau amounts in cortex (Figure 6g) and hippocampus (Supplemental Figure 8c) of 6-day-old mice.
Vitamin K2 decreased amounts of Tau-PS356 and Tau-PS202/PT205 (Figure 7a–c). Specifically, under baseline non-anesthetized conditions, we found vitamin K2 decreased the amounts of Tau-PS202/PT205 as compared to vehicle, but the difference did not reach to statistically significant (non-anesthetized, vitamin K2: 0.4 ± 0.2 arbitrary unit versus non-anesthetized, vehicle: 0.7 ± 0.2 arbitrary unit, P = 0.150] (Figure 7a and 7c). In contrast, the sevoflurane anesthesia-induced increases in Tau-PS202/PT205 amounts were significantly attenuated by vitamin K2 (sevoflurane, vitamin K2: 0.3 ± 0.2 arbitrary unit versus sevoflurane, vehicle: 1.4 ± 0.4 arbitrary unit, P = 0.011) in cortex (Figure 7a and 7c) and hippocampus (Supplemental Figure 8a–b) of the neonatal mice.
Figure 7. Rescuing effects of vitamin K2 on Tau phosphorylation and cognitive impairment in neonatal mice from both sexes.
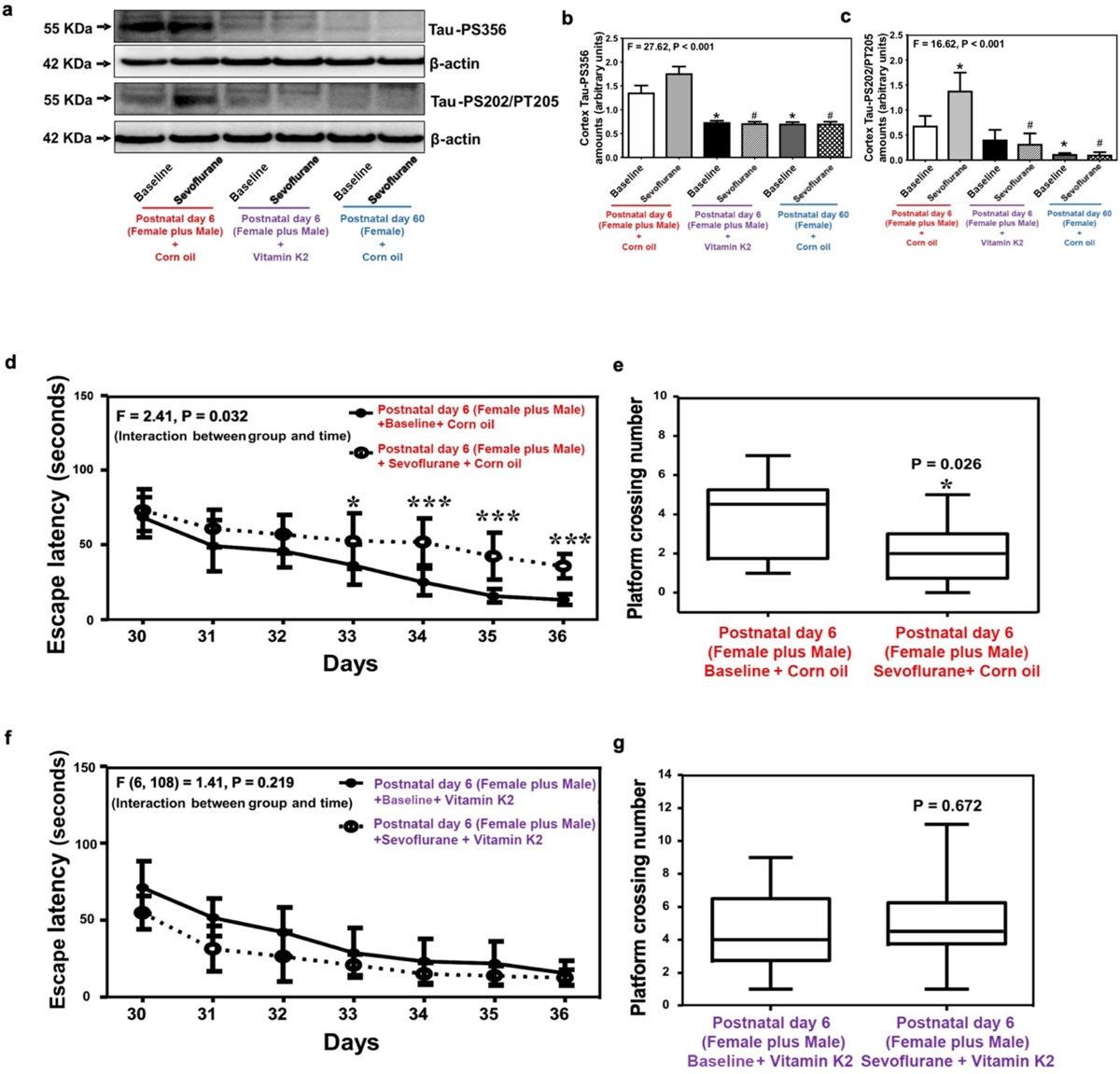
(a) Amounts of Tau-PS356 and Tau-PS202/PT205 in the cortex of neonatal mice from both sexes and female reference group mice. Summary of amounts of Tau-PS356 (b) and Tau-PS202/PT205 (c) (n=6 mice/group). Escape latency (d) and platform crossing number (e) of Morris water maze test following vehicle treatment for sevoflurane anesthesia and baseline groups (n=10 mice/group). Escape latency (f) and platform crossing number (g) of Morris water maze test following vitamin K2 treatment for sevoflurane anesthesia and baseline groups (n=10 mice/group). Data are presented as mean ± standard deviation (SD). All data are quantified and expressed as arbitrary units or real numbers compared to baseline group (non-anesthetized mice). PS, phosphorylated serine; PT, phosphorylated threonine. One-way ANOVA: (b) and (c). Two-way ANOVA with repeated measurement: (d) and (f). Mann-Whitney test: (e) and (g). *P<0.05; #P<0.05.
The amounts of Tau-PS356 and Tau-PS202/PT205 in the cortex or hippocampus of neonatal mice from both sexes treated with vitamin K2 were comparable to those in the cortex of female reference group mice treated with vehicle in baseline non-anesthetized conditions and following sevoflurane exposure (Figure 7a–c).
Further, sevoflurane anesthesia induced cognitive impairment in Morris water maze tests in neonatal mice from both sexes pre-treated with vehicle (Figure 7d–e) but not in the neonatal mice pre-treated with vitamin K2 as compared to the baseline (Figure 7f–g).
Brain tissues of neonatal and adult mice have different mitochondrial metabolism
Given differences in brain ATP concentrations between neonatal mice from both sexes and reference group mice, we investigated the underlying mechanism by determining baseline mitochondrial metabolism in brain tissues of neonatal mice from both sexes and the reference group mice. Hippocampus mitochondrial metabolism in neonatal mice from both sexes were lower than those in female reference group mice, e.g., basal respiration: 74.8 ± 14.1 pmoles/minute versus 169.6 ± 15.3 pmoles/minute, P < 0.001 (Figure 8a–d), and male reference group mice (Figure 8e–h). There were no significant differences in hippocampus mitochondrial metabolism between female and male postnatal day 60 mice (Supplemental Figure 9a–d).
Figure 8. Brain mitochondrial metabolism in neonatal and adult mice.

Overall lower oxygen consumption rate (a), basal respiration (b), ADP respiration (c), and maximal respiration (d) in the hippocampus of neonatal mice from both sexes than female reference group mice (n=6, Student’s t-test). Overall lower oxygen consumption rate (e), basal respiration (f), ADP respiration (g), and maximal respiration (h) in the hippocampus of neonatal mice from both sexes than male reference group mice (n=6, Student’s t-test). Data are presented as mean ± standard deviation (SD). All data are quantified and expressed as real numbers compared to reference group mice (adult mice). Student’s t-test, ***P<0.001.
Discussion
This study provides new mechanistic insights into the age-dependent effects of sevoflurane on cognitive function. We found that 6-day-old neonatal mice have higher brain amounts of Tau and phosphorylated Tau proteins than adult mice both under baseline non-anesthetized conditions and following repeated exposure to sevoflurane. Moreover, neonatal mice had less baseline brain ATP concentrations and exhibited lower mitochondrial metabolism than adult mice. Finally, both Nuak1 inhibitor (HTH-01–015) and energy enhancer (vitamin K2) attenuated sevoflurane-induced Tau phosphorylation and cognitive impairment in neonatal mice from both sexes. These data suggest existence of a potential cascade of brain mitochondria-ATP-Nuak1-Tau and that higher baseline Tau amounts in brain tissues of neonatal mice contribute to age-dependent differences in sevoflurane anesthesia-induced Tau phosphorylation and cognitive function changes between neonatal and adult mice.
Previous studies demonstrate that anesthesia with one minimum alveolar concentration isoflurane for 4 h in postnatal day 7 rats induces neuronal death at 12 h after anesthesia in both males and females but only impairs recognition of objects and social memory deficits in male rats at postnatal day 3833 These results indicate there are potential sex difference in anesthesia neurotoxicity between male and female rats. Moreover, isoflurane anesthesia at postnatal day 4 induces greater neurotoxicity and neurobehavioral deficits than at postnatal day 7 in female but not male mice, suggesting sex- and age-dependent anesthesia neurotoxicity in developing brains.34 This finding is consistent with other studies showing sex-dependent anesthesia neurotoxicity in developing brains.35
In the present study, however, we did not observe sex-dependent changes as evidenced by higher amounts of Tau, Nuak1, and Tau-PS356 but lower concentrations of ATP and mitochondrial metabolism in the brains of both neonatal mice from both sexes (postnatal day 6) from both sexes as compared to adult (postnatal day 60) female or male mice. Moreover, there were no significant differences in the amounts of Tau, Nuak1, Tau-PS356, ATP concentration, mitochondrial metabolism, and cognitive function between female and male postnatal day 60 mice in the baseline and sevoflurane anesthesia groups. The reason for these differences between our results and previous studies is not known currently. However, previous studies used younger rodents (e.g., 4-day-old) and identified the sex of these rodents, whereas we used 6-day-old mice from both sexes. Nevertheless, our findings promote future research to determine if there is sex-dependent developmental anesthesia neurotoxicity in mice.
Greater Nuak1 amounts were not due to more production but rather likely due to less protein metabolism, as evidenced by lower amounts of phosphorylated Nuak1 in brains of neonatal mice from both sexes as compared to adult mice. ATP plays an important role in protein phosphorylation.36,37 In this regard, our results show that neonatal mice from both sexes had less baseline brain ATP and mitochondrial metabolism than adult mice. Nevertheless, greater Nuak1 amounts could also be due to other reasons, including lesser Nuak1 degradation. Future studies will seek to test this hypothesis by systematically comparing amounts of all Nuak1 metabolites in brain tissues between neonatal and adult mice by using mass spectrometry for screening and western blot for confirmation.
The Nuak1 inhibitor HTH-01–015 and vitamin K2 attenuated higher amounts of Tau-PS356, T22, and pTau (baseline and sevoflurane-induced) in the brains of neonatal mice from both sexes as well as sevoflurane-induced cognitive impairment. These data further suggest that differences in brain Nuak1 and energy amounts between neonatal and adult mice contribute to differences in brain Tau phosphorylation and cognitive function following administration of sevoflurane anesthesia. Notably, there were comparable amounts of brain Tau-PS356 and T22 between neonatal mice from both sexes treated with HTH-01–015 or vitamin K2 and adult mice treated with vehicle. These findings suggest that targeting Nuak1 and energy may reduce the difference between neonatal and adult mice in relation to vulnerability to anesthesia-induced cognitive impairment in neonatal mice from both sexes.
HTH-01–015 did not increase brain ATP concentrations in neonatal mice from both sexes compared to vehicle, although the mitochondrial electron carrier and energy enhancer vitamin K2 increased brain ATP concentrations in neonatal mice from both sexes. These data suggest that changes in ATP concentrations occur before alterations of Nuak1 amounts.
Moreover, vitamin K2 also attenuated higher amounts of Tau-PS356, T22, pTau, and Tau- and T22-positive cells in the brains of neonatal mice from both sexes compared to vehicle. Further, vitamin K2 mitigated sevoflurane anesthesia-induced brain Tau phosphorylation and cognitive impairment in neonatal mice from both sexes. These data further suggest that deprivation of brain energy in neonatal mice from both sexes is one of the underlying mechanisms by which neonatal mice from both sexes become more vulnerable to Tau phosphorylation and cognitive impairment following administration of sevoflurane anesthesia.
Interestingly, neither HTH-01–015 nor vitamin K2 decreased total Tau amounts in the brains of postnatal day 6 mice. This could be attributed to the fact that the half-life of Tau is 9.7 days38 and that we only harvested brain tissues three days after the first treatment of HTH-01–015 or vitamin K2. Neonatal mice from both sexes may have lower concentrations of ATP and mitochondrial metabolism as compared to adult mice due to less glucose metabolism,39 but this still remains to be determined in future studies.
Using mice overexpressing the human Tau transgene (hTau mice), Polydoro et al. reported that 12-month-old hTau mice have higher incidences of tauopathy, impaired synaptic transmission, and neurobehavioral deficits than 4-month-old hTau mice.40 Consistently, our data show age-dependent Tau phosphorylation and cognitive impairment following administration of sevoflurane anesthesia. However, the current studies further illustrate that such age-dependent changes in Tau phosphorylation and cognitive function could be due to the age-dependent cascade of mitochondria-ATP-Nuak1-Tau (Supplemental Figure 10), with neonatal mice from both sexes more vulnerable to development of Tau phosphorylation and cognitive impairment. Future studies are needed to further test this hypothesis using hTau mice.
Despite strengths, our studies also have several limitations. First, we did not use western blot to confirm other Tau phosphorylation sites screened by mass spectrometry. However, the purpose of the current studies was to assess whether differences in amounts of brain Tau, Nuak1, ATP concentration, and mitochondrial metabolism between neonatal and adult mice contributed to differences of sevoflurane-induced Tau phosphorylation and cognitive impairment. We chose Tau-PS202/Tau-PT205 as representative of pTau for this purpose. Second, we did not identify male and female neonatal mice due to difficulties identifying sex in neonatal mice. However, our objective was to reveal the underlying mechanism by which neonatal mice were more vulnerable to development of Tau phosphorylation and cognitive impairment following sevoflurane anesthesia. Looking forward, we will use the established system to assess potential sex differences in Tau phosphorylation and cognitive function in neonatal mice. Third, we determined age-dependent changes in Tau metabolism, its upstream mechanism and downstream consequences in the whole cortex and hippocampus rather than in subregions of the hippocampus and cortex, so it is unknown if age-dependent anesthesia neurotoxicity is dependent on specific brain regions. However, the objective of the present study was to compare general baseline amounts of Tau, ATP, mitochondrial metabolism, etc. in brain tissues of neonatal and adult mice. Fourth, the addition of new mice in our experiments, which had differences in birth time and mother, litter, and experimental condition, add potential bias and confounding to our outcomes. Finally, mice in both baseline and anesthesia groups received 60% oxygen, leading to potential confounding influences due to effects of released oxygen free radical. However, previous studies using the same anesthesia (3% sevoflurane plus 60% oxygen) also illustrated age-dependent anesthesia neurotoxicity,14 so we used this established system to demonstrate age-dependent Tau metabolism and associated changes.
In conclusion, our results indicate that higher brain Tau concentrations and lower activity of the mitochondria-ATP-Nuak1-Tau phosphorylation cascade in neonatal compared to adult mice may serve as an underlying mechanism of age-dependent Tau phosphorylation and cognitive impairment following administration of sevoflurane anesthesia in mice.
Supplementary Material
Acknowledgment:
This research was supported by HD086977 from National Institutes of Health, Bethesda, MD to Zhongcong Xie. These studies were performed in Massachusetts General Hospital and Harvard Medical School and are attributed to the Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital and Harvard Medical School.
Footnotes
COMPETING FINANCIAL INTERESTS: The authors declare no competing financial interests. Dr. Zhongcong Xie is a consultant for Novartis, Baxter (invited speaker), Tongji University, and Shanghai Jiaotong University.
Conflict of interest: Dr. Zhongcong Xie provides consulting service to Shanghai Jiaotong University, Tongji University, Baxter (invited speaker), and Novartis. The authors do not have equity interests and patent-licensing arrangement.
References
- 1.Sun L: Early childhood general anaesthesia exposure and neurocognitive development. Br J Anaesth 2010; 105 Suppl 1: i61–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vutskits L, Xie Z: Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci 2016; 17: 705–717 [DOI] [PubMed] [Google Scholar]
- 3.Warner DO, Zaccariello MJ, Katusic SK, Schroeder DR, Hanson AC, Schulte PJ, Buenvenida SL, Gleich SJ, Wilder RT, Sprung J, Hu D, Voigt RG, Paule MG, Chelonis JJ, Flick RP: Neuropsychological and Behavioral Outcomes after Exposure of Young Children to Procedures Requiring General Anesthesia: The Mayo Anesthesia Safety in Kids (MASK) Study. Anesthesiology 2018; 129: 89–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Leary JD, Janus M, Duku E, Wijeysundera DN, To T, Li P, Maynes JT, Crawford MW: A Population-based Study Evaluating the Association between Surgery in Early Life and Child Development at Primary School Entry. Anesthesiology 2016; 125: 272–9 [DOI] [PubMed] [Google Scholar]
- 5.Graham MR, Brownell M, Chateau DG, Dragan RD, Burchill C, Fransoo RR: Neurodevelopmental Assessment in Kindergarten in Children Exposed to General Anesthesia before the Age of 4 Years: A Retrospective Matched Cohort Study. Anesthesiology 2016; 125: 667–677 [DOI] [PubMed] [Google Scholar]
- 6.Cao W, Pavlinec C, Gravenstein N, Seubert CN, Martynyuk AE: Roles of aldosterone and oxytocin in abnormalities caused by sevoflurane anesthesia in neonatal rats. Anesthesiology 2012; 117: 791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan S, Xu C, Zhu W, Willis J, Seubert CN, Gravenstein N, Sumners C, Martynyuk AE: Endocrine and neurobehavioral abnormalities induced by propofol administered to neonatal rats. Anesthesiology 2014; 121: 1010–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willis J, Zhu W, Perez-Downes J, Tan S, Xu C, Seubert C, Gravenstein N, Martynyuk A: Propofol-induced electroencephalographic seizures in neonatal rats: the role of corticosteroids and gamma-aminobutyric acid type A receptor-mediated excitation. Anesth Analg 2015; 120: 433–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu C, Tan S, Zhang J, Seubert CN, Gravenstein N, Sumners C, Vasilopoulos T, Martynyuk AE: Anesthesia with sevoflurane in neonatal rats: Developmental neuroendocrine abnormalities and alleviating effects of the corticosteroid and Cl(−) importer antagonists. Psychoneuroendocrinology 2015; 60: 173–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Xu C, Puentes DL, Seubert CN, Gravenstein N, Martynyuk AE: Role of Steroids in Hyperexcitatory Adverse and Anesthetic Effects of Sevoflurane in Neonatal Rats. Neuroendocrinology 2016; 103: 440–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Ju L, Yang C, Xue J, Setlow B, Morey TE, Gravenstein N, Seubert CN, Vasilopoulos T, Martynyuk AE: Effects of combined brief etomidate anesthesia and postnatal stress on amygdala expression of Cl(−) cotransporters and corticotropin-releasing hormone and alcohol intake in adult rats. Neurosci Lett 2018; 685: 83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia M, Liu WX, Yang JJ, Xu N, Xie ZM, Ju LS, Ji MH, Martynyuk AE, Yang JJ: Role of histone acetylation in long-term neurobehavioral effects of neonatal Exposure to sevoflurane in rats. Neurobiol Dis 2016; 91: 209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ju LS, Yang JJ, Morey TE, Gravenstein N, Seubert CN, Resnick JL, Zhang JQ, Martynyuk AE: Role of epigenetic mechanisms in transmitting the effects of neonatal sevoflurane exposure to the next generation of male, but not female, rats. Br J Anaesth 2018; 121: 406–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, Sun D, Baxter MG, Zhang Y, Xie Z: Selective Anesthesia-induced Neuroinflammation in Developing Mouse Brain and Cognitive Impairment. Anesthesiology 2013; 118: 502–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tao G, Zhang J, Zhang L, Dong Y, Yu B, Crosby G, Culley DJ, Zhang Y, Xie Z: Sevoflurane induces tau phosphorylation and glycogen synthase kinase 3beta activation in young mice. Anesthesiology 2014; 121: 510–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu H, Liufu N, Dong Y, Xu G, Zhang Y, Shu L, Soriano SG, Zheng H, Yu B, Xie Z: Sevoflurane Acts on Ubiquitination-Proteasome Pathway to Reduce Postsynaptic Density 95 Protein Levels in Young Mice. Anesthesiology 2017; 127: 961–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu G, Lu H, Dong Y, Shapoval D, Soriano SG, Liu X, Zhang Y, Xie Z: Coenzyme Q10 reduces sevoflurane-induced cognitive deficiency in young mice. Br J Anaesth 2017; 119: 481–491 [DOI] [PubMed] [Google Scholar]
- 18.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW: A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A 1975; 72: 1858–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Querfurth HW, LaFerla FM: Alzheimer’s disease. N Engl J Med 2010; 362: 329–44 [DOI] [PubMed] [Google Scholar]
- 20.Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR: LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J 2004; 23: 833–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lasagna-Reeves CA, de Haro M, Hao S, Park J, Rousseaux MW, Al-Ramahi I, Jafar-Nejad P, Vilanova-Velez L, See L, De Maio A, Nitschke L, Wu Z, Troncoso JC, Westbrook TF, Tang J, Botas J, Zoghbi HY: Reduction of Nuak1 Decreases Tau and Reverses Phenotypes in a Tauopathy Mouse Model. Neuron 2016; 92: 407–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerjee S, Buhrlage SJ, Huang HT, Deng X, Zhou W, Wang J, Traynor R, Prescott AR, Alessi DR, Gray NS: Characterization of WZ4003 and HTH-01–015 as selective inhibitors of the LKB1-tumour-suppressor-activated NUAK kinases. Biochem J 2014; 457: 215–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vos M, Esposito G, Edirisinghe JN, Vilain S, Haddad DM, Slabbaert JR, Van Meensel S, Schaap O, De Strooper B, Meganathan R, Morais VA, Verstreken P: Vitamin K2 is a mitochondrial electron carrier that rescues pink1 deficiency. Science 2012; 336: 1306–10 [DOI] [PubMed] [Google Scholar]
- 24.Li J, Lin JC, Wang H, Peterson JW, Furie BC, Furie B, Booth SL, Volpe JJ, Rosenberg PA: Novel role of vitamin k in preventing oxidative injury to developing oligodendrocytes and neurons. J Neurosci 2003; 23: 5816–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onodera K, Shinoda H, Zushida K, Taki K, Kamei J: Antinociceptive effect induced by intraperitoneal administration of vitamin K2 (menatetrenone) in ICR mice. Life Sci 2000; 68: 91–7 [DOI] [PubMed] [Google Scholar]
- 26.Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, Frosch MP, Crosby G, Tanzi RE: The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol 2008; 64: 618–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z: Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol 2012; 71: 687–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ting L, Rad R, Gygi SP, Haas W: MS3 eliminates ratio distortion in isobaric multiplexed quantitative proteomics. Nat Methods 2011; 8: 937–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards A, Haas W: Multiplexed Quantitative Proteomics for High-Throughput Comprehensive Proteome Comparisons of Human Cell Lines. Methods Mol Biol 2016; 1394: 1–13 [DOI] [PubMed] [Google Scholar]
- 30.McAlister GC, Nusinow DP, Jedrychowski MP, Wuhr M, Huttlin EL, Erickson BK, Rad R, Haas W, Gygi SP: MultiNotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal Chem 2014; 86: 7150–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erickson BK, Jedrychowski MP, McAlister GC, Everley RA, Kunz R, Gygi SP: Evaluating multiplexed quantitative phosphopeptide analysis on a hybrid quadrupole mass filter/linear ion trap/orbitrap mass spectrometer. Anal Chem 2015; 87: 1241–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP: A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat Biotechnol 2006; 24: 1285–92 [DOI] [PubMed] [Google Scholar]
- 33.Lee BH, Chan JT, Kraeva E, Peterson K, Sall JW: Isoflurane exposure in newborn rats induces long-term cognitive dysfunction in males but not females. Neuropharmacology 2014; 83: 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasaki Russell JM, Chinn GA, Maharjan D, Eichbaum Y, Sall JW: Female rats are more vulnerable to lasting cognitive impairment after isoflurane exposure on postnatal day 4 than 7. Br J Anaesth 2019; 122: 490–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ju LC, Yang JJ, Morey TE, Gravenstein N, Seubert CN, Resnick JL, Zhang JQ, Martynyuk AE: Role of epigenetic mechanisms in transmitting the effects of neonatal sevoflurane exposure to the next generation of male, but not female, rats. British Journal of Anaesthesia 2018; 121: 406–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Cole PA: Catalytic mechanisms and regulation of protein kinases. Methods Enzymol 2014; 548: 1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian H: Phosphorylation energy hypothesis: open chemical systems and their biological functions. Annu Rev Phys Chem 2007; 58: 113–42 [DOI] [PubMed] [Google Scholar]
- 38.Yamada K, Patel TK, Hochgrafe K, Mahan TE, Jiang H, Stewart FR, Mandelkow EM, Holtzman DM: Analysis of in vivo turnover of tau in a mouse model of tauopathy. Mol Neurodegener 2015; 10: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacob Z, Li H, Makaryus R, Zhang S, Reinsel R, Lee H, Feng T, Rothman DL, Benveniste H: Metabolomic profiling of children’s brains undergoing general anesthesia with sevoflurane and propofol. Anesthesiology 2012; 117: 1062–71 [DOI] [PubMed] [Google Scholar]
- 40.Polydoro M, Acker CM, Duff K, Castillo PE, Davies P: Age-dependent impairment of cognitive and synaptic function in the htau mouse model of tau pathology. J Neurosci 2009; 29: 10741–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


