Abstract
Rationale:
Unproven theories abound regarding the long-range uptake and endocrine activity of extracellular blood-borne microRNAs (miRNAs) into tissue. In pulmonary hypertension (PH), microRNA-210 (miR-210) in pulmonary endothelial cells promotes disease, but its activity as an extracellular molecule is incompletely defined.
Objective:
We investigated whether chronic and endogenous endocrine delivery of extracellular miR-210 to pulmonary vascular endothelial cells promotes PH.
Methods and Results:
Using miR-210 replete (WT) and knockout (KO) mice, we tracked blood-borne miR-210 using bone marrow transplantation (BMT) and parabiosis (conjoining of circulatory systems). With BMT, circulating miR-210 was derived predominantly from bone marrow. Via parabiosis during chronic hypoxia to induce miR-210 production and PH, miR-210 was undetectable in KO-KO mice pairs. However, in plasma and lung endothelium, but not smooth muscle or adventitia, miR-210 was observed in KO mice of WT-KO pairs. This was accompanied by down-regulation of miR-210 targets ISCU1/2 and COX10, indicating endothelial import of functional miR-210. Via hemodynamic and histologic indices, KO-KO pairs were protected from PH, while KO mice in WT-KO pairs developed PH. In particular, pulmonary vascular engraftment of miR-210-positive interstitial lung macrophages was observed in KO mice of WT-KO pairs. To address whether engrafted miR-210-positive myeloid or lymphoid cells contribute to paracrine miR-210 delivery, we studied miR-210 KO mice parabiosed with miR-210 WT; Cx3cr1 KO mice (deficient in myeloid recruitment) or miR-210 WT; Rag1 KO mice (deficient in lymphocytes). In both pairs, miR-210 KO mice still displayed miR-210 delivery and PH, thus demonstrating a pathogenic endocrine delivery of extracellular miR-210.
Conclusions:
Endogenous blood-borne transport of miR-210 into pulmonary vascular endothelial cells promotes PH, offering fundamental insight into the systemic physiology of miRNA activity. These results also describe a platform for RNA-mediated crosstalk in PH, providing an impetus for developing blood-based miR-210 technologies for diagnosis and therapy in this disease.
Subject Terms: Biomarkers, Cardiovascular Disease, Pulmonary Hypertension, Vascular Biology
Keywords: Circulating microRNAs, parabiosis, pulmonary vascular endothelium, interstitial lung macrophage, lymphocytes, vascular disease, pulmonary hypertension
Graphical Abstract
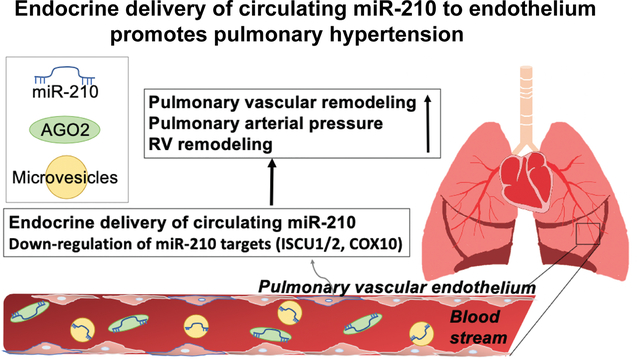
INTRODUCTION
MicroRNAs (miRNAs) are small, conserved, non-coding RNAs that engage messenger transcript target sequences intracellularly and negatively regulate gene expression1. Circulating, extracellular miRNAs expressed in the bloodstream have been described2 across numerous examples of human health and disease3, 4. While these extracellular isoforms have been explored as potential diagnostic and prognostic biomarkers5, advancing evidence has indicated that delivery of these factors, either packaged in microvesicles6 or as RNA-protein complexes7, to recipient tissue can result in canonical miRNA-mediated control of gene expression5. Prior studies have demonstrated uptake in cell culture8 as well as in artificial supraphysiologic contexts in vivo5, 9, but proof of long-range endocrine delivery of these molecules has been elusive10 and confounded by multiple non-miRNA-dependent variables11. In fact, the exceedingly low levels of miRNA expression in microvesicles12 (1 copy per 100–1000 microvesicles for the most abundant miRNAs) have promoted skepticism whether endogenous extracellular miRNAs participate in enough delivery to have disease-modifying activities as endocrine factors.
In the vasculature, an anatomic rationale exists for endocrine delivery of extracellular miRNAs, since the endothelial layer is exposed most directly and chronically to these circulating factors. Yet, in both the peripheral and pulmonary vasculature, evidence is sparse regarding the endogenous in vivo roles of extracellular miRNA communication. Notably, in existing data, paracrine transport more often has been proposed between neighboring smooth muscle and endothelial cells13 or between innate immune myeloid and endothelial cells14, 15. But again, the in vivo relevance of these vascular communication axes has not been fully described. In particular, pathogenesis of pulmonary hypertension (PH), a deadly vascular disease caused by complex and enigmatic processes, has been directly linked to miRNA pathobiology1. But, historically, beyond the dysregulation of soluble inflammatory cytokines and locally produced factors controlling vasomotor tone16, PH has never been viewed as primarily controlled by endocrine mechanisms. Thus, if long-range extracellular miRNA transport to the pulmonary vasculature does occur, it would represent a paradigm shift from the narrower cardiopulmonary view of PH pathogenesis.
The hypoxia-inducible microRNA-210 (miR-210) has been implicated as a causative factor promoting multiple subtypes of PH, namely pulmonary arterial hypertension (PAH or Group 1 PH as designated by the World Symposium on Pulmonary Hypertension, WSPH) as well as PH due to hypoxic lung disease (WSPH Group 3 PH)17. By repressing the iron-sulfur (Fe-S) cluster assembly proteins ISCU1/218, miR-210 acts as an intracellular pathogenic factor in pulmonary arterial endothelial cells (PAECs) to induce metabolic dysregulation and PH in vivo17. Circulating miR-210 is elevated in human plasma in physiologic and pathologic conditions of altered oxygen demand and delivery19. Prolyl-hydroxylation of Argonaute 2 (AGO2), as controlled by hypoxia versus reoxygenation, in part controls the packaging of circulating miR-210 and consequent transport and activity19. Furthermore, extracellular miR-210 can be taken up by cultured PAECs with reciprocal downregulation of ISCU1/2, indicating import of functionally active miR-210 by this cell type. Transfer of miR-210 from one cellular compartment to another via microparticle delivery has also been reported in vitro in cultured tumor cells8. The endocrine transport of endogenous miR-210 in vivo, however, has been poorly characterized.
Based on the presence of miR-210 throughout various anatomic compartments as well as the in vitro transport capacity of this miRNA, it is possible that the pathogenic and vascular functions of miR-210 in PH may rely on extrapulmonary transport in the blood to the pulmonary vessels. This could be accomplished via long-range endocrine uptake of extracellular miR-210 or through engraftment of miR-210-positive hematologic cells leading to paracrine transport. Regarding the latter, a growing literature has emphasized the importance of interstitial macrophages in the inflammatory pathophenotype and overall pathogenesis of PH20–23. Most recently, the pulmonary infiltration of blood-borne monocytes and differentiation to interstitial macrophages in the pulmonary vasculature have been reported as key events in the inflammatory cascade driving PH23. Indeed, evidence exists for the importance of miR-210 in cells of innate24 and acquired immunity25–27. Thus, we aimed to determine whether PH depends critically on such blood-borne transport of pathogenic miR-210 and to delineate the contributions of endocrine transport, independent of any paracrine delivery from engrafted inflammatory cells.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request. Details on further experimental methods are provided in an online data supplement.
Study approval.
All animal experiments were approved by the University of Pittsburgh School of Medicine (DLAR).
RESULTS
Chronic delivery of blood-borne miR-210 to pulmonary vascular endothelial cells in vivo.
We sought to determine whether chronic exposure to blood-borne miR-210 allows for delivery and pathogenic activity in pulmonary vascular cell types. First, to define the relative contribution to the pool of plasma miR-210 from hematopoietic bone marrow vs. non-hematopoietic/non-bone marrow contents, we performed bone marrow transplantation (BMT) experiments between mice that are replete (WT) and deficient (KO)25 for miR-210 (Fig. 1A). We found the major strand (miR-210–3p, denoted as miR-210 throughout this text) (Fig.1B) and passenger strand (miR-210–5p) (Fig. IA) of miR-210 were circulating in plasma of KO mice transplanted with WT bone marrow. For miR-210, we found 130,709 ± 27,503 (mean ± S.E.M) circulating extracellular copies per μl of plasma in WT mice transplanted with WT bone marrow. However, only 9,305 ± 1,795 copies of miR-210/μl of plasma were observed in WT mice transplanted with KO bone marrow (Fig. 1B). Therefore, we calculated that over 90% of circulating miR-210 was derived from bone marrow cells, while less than 10% of circulating miR-210 were released from other tissues and independent of the influence of miR-210-positive bone marrow cells (Fig. 1B).
Figure 1. A majority of circulating, steady-state hypoxia-induced miR-210 is derived from bone marrow cells.
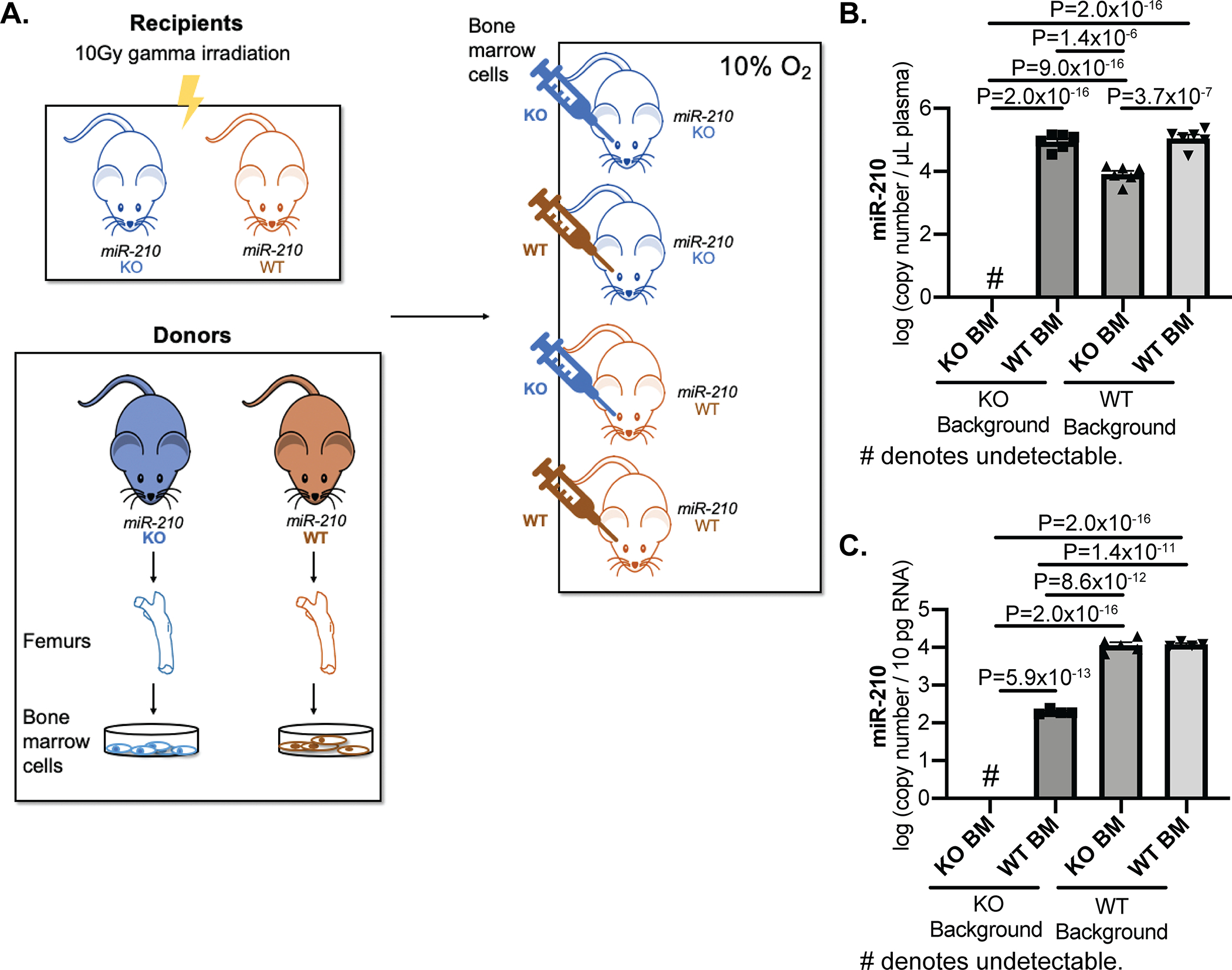
(A) At 12 weeks post-bone marrow transplantation of miR-210 (WT) and miR-210 (KO) mice with WT or KO bone marrow, mice were exposed to chronic hypoxia (10% O2) for 3 weeks to induce miR-210 up-regulation. (B-C) By RT-qPCR, circulating extracellular miR-210 (B, N=5,6,6,6 mice) and intracellular miR-210 (C, N=4,4,5,4 mice) were detected and quantified in plasma (B) and CD31+/CD45− lung endothelial cells (C) of WT mice and KO mice transplanted with WT or KO bone marrow. (One-way ANOVA with post-hoc Bonferroni testing was performed.)
These data were corroborated by our observation of elevated levels of plasma miR-210 in KO mice transplanted with WT bone marrow as compared with KO bone marrow (Fig. 1B). Because of the known pathogenic importance of endogenous miR-210 activity in PAECs17 for PH, we focused on endothelial cells specifically as the recipient vascular cell type. Correspondingly, we quantified miR-210 in the CD31+/CD45− lung endothelial cells of KO or WT mice after BMT. First, we studied a context where hematopoietic bone marrow was unable to contribute to plasma miR-210. We found that miR-210 endothelial content in WT mice transplanted with KO BM was similar to transplantation with WT BM (Fig. 1C), consistent with the notion that in this context endothelial miR-210 originated from either or both of two sources: circulating miR-210 derived from non-hematopoietic/non-bone marrow contents or endogenously produced miR-210 in lung endothelial cells themselves. Second, we studied a context where hematopoietic bone marrow was the only source of plasma miR-210. In this case, we also found an increase of miR-210 in lung endothelial cells of KO mice receiving WT (vs. KO) bone marrow (Fig. 1C), mirroring the increase of miR-210 in plasma (Fig. 1B). While hematopoietic bone-marrow cell-derived miR-210 did not entirely compensate for the levels of WT miR-210 endothelial expression, these data demonstrate that bone marrow contributes substantially to the plasma levels of miR-210 and bone marrow miR-210 can be delivered to the pulmonary vascular endothelium.
To expose mice to endogenous levels of blood-borne miR-210 chronically and more directly assess for endocrine transport, we utilized an in vivo parabiosis platform to conjoin the circulatory systems of two mice in the context of miR-210 +/+ (WT) and miR-210 −/− (KO)25 partners (Fig. 2A). One-week post-surgery, mouse pairs were exposed to chronic hypoxia (10% O2) for 3 weeks to induce miR-210 up-regulation. To determine if endogenous blood-borne miR-210 can be delivered to the vasculature in vivo, miR-210 expression was quantified by RT-qPCR in plasma and in isolated CD31+/CD45− lung endothelial cells as well as by in situ staining in CD31-positive pulmonary arteriolar endothelium. By RT-qPCR, extracellular and intracellular miR-210 isoforms in KO-KO mice pairs were negligible (Fig. 2B, 2D, IB–C). In contrast, when paired with WT mice, extracellular levels of miR-210 isoforms in plasma of KO partners were readily detected and comparable with WT-WT pairs (Fig. 2B, IB). Consistent with prior findings in vitro19, immunoprecipitation revealed that released circulating miR-210 is transported, at least in part, in a RNA-protein complex with AGO2, a major factor involved in the intracellular RNA induced silencing complex (RISC)28 (Fig. 2C). Similarly, intracellular endothelial miR-210 in the KO partner of KO-WT pairs was observed at substantial levels, albeit lower than in endothelial cells of the WT partners (Fig. 2D).
Figure 2. Chronic conjoining of the circulatory systems in mice via parabiosis leads to delivery of blood-borne miR-210 to pulmonary arterial endothelial cells in vivo.
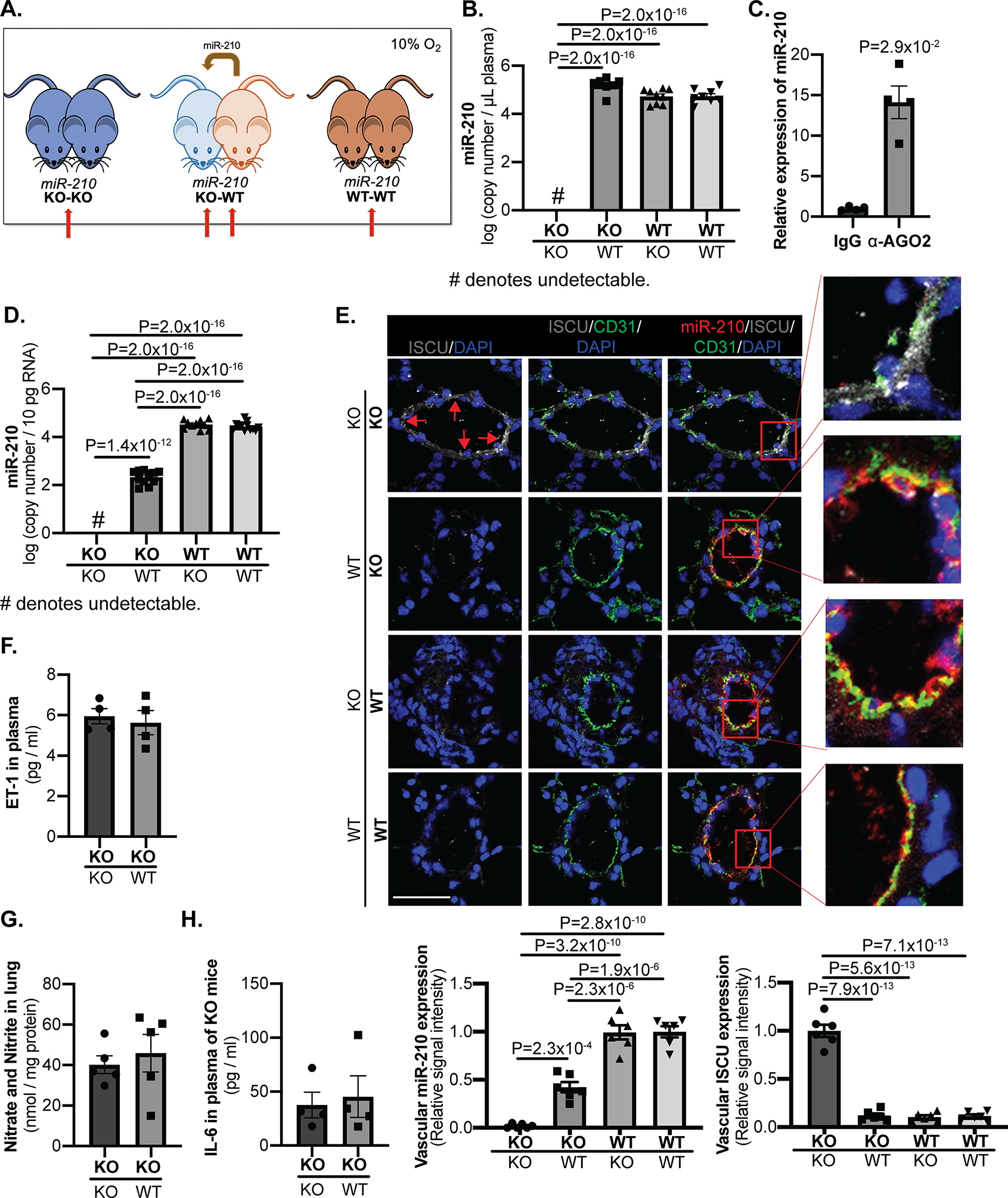
(A) An in vivo parabiosis platform allowed for the conjoining of the circulatory systems of two mice in the context of miR-210 +/+ (WT) and miR-210 −/− (KO) partners. One-week post-surgery, mouse pairs were exposed to chronic hypoxia (10% O2) for 3 weeks to induce endogenous miR-210 up-regulation. (Red arrows denote comparison groups.) (B-D) As assessed by RT-qPCR in plasma (B, N=8,7,9,8 mice) and in CD31+/CD45− lung endothelial cells (D, N=12,11,10,12 mice), while miR-210 expression was negligible in KO-KO pairs, extracellular miR-210 level in KO partners of KO-WT pairs was increased to comparable level as WT-WT pairs whereas intracellular miR-210 in those mice was readily detected but still less than that of WT partners and WT-WT pairs. Extracellular miR-210 in plasma was found to be co-immunoprecipitated specifically in the presence of α-Argonaute 2 (AGO2) as compared with control IgG (C, N=4 mice/group). (E) By in situ staining of miR-210 (red), its target proteins ISCU1/2 (grey, pointed out by red arrows), and the endothelial marker CD31 (green), miR-210 expression in the KO partners of KO-WT pairs was detected specifically in pulmonary arteriolar endothelium (yellow, micrograph inset), accompanied by a reciprocal decrease in ISCU1/2 expression in these miR-210-positive cells, as compared with KO-KO pairs (N=6 mice/group, average of N=10–12 vessels/mice. Scale bar denotes 50 μm.). (F-H) In parallel, there was no significant difference of endothelin-1 content in plasma (F, N=4 mice/group), nitrate and nitrite content in lungs (G, N=5 mice/group) or inflammatory cytokine IL-6 content in plasma (H, N=4 mice/group) between KO partners of KO-WT pairs with KO-KO pair. (Data are presented as mean ± SEM. In x-axes of (B,D,E,F,G,H) bold font denotes parabionts being studied, whereas regular font denotes partner parabionts. Mann Whitney U test for C,F,G,H and one-way ANOVA with post-hoc Bonferroni testing were performed for other panels.)
To better characterize the specificity of delivery, in situ stain of pulmonary arterioles in cross section (Fig. 2E, IIA) and vessels in longitudinal section (Fig. IIB) demonstrated that such miR-210 expression in the KO partner of KO-WT pairs was evident specifically and consistently in the endothelium, but not in the smooth muscle or adventitial layers (Fig. IIA). These findings were corroborated by RT-qPCR of cells isolated from large pulmonary arteries outside the lung, where we found detectable miR-210 in CD31+/CD45− endothelial cells but not in CD31- non-endothelial cells (Fig. IIC), thus offering evidence against predominant paracrine transfer of miR-210 from smooth muscle/adventitia towards vascular endothelial cells in this context. Furthermore, miR-210 staining was consistent across the endothelium of small and large pulmonary arteries as well as in pulmonary veins (Fig. IID). RT-qPCR quantification corroborated this finding, demonstrating similar levels of miR-210 in CD31+/CD45− endothelial cells from large pulmonary arteries outside the lung and from peripheral lungs (Fig. IIE). Thus, we found no evidence of distinct “hot spots” of endothelial delivery that would be unique sources for instigating predominantly paracrine transport to other areas of the endothelium. Notably, while detectable in the plasma, neither the passenger strand miR-210–5p nor precursor miR-210 molecule were observed in lung endothelial cells of the KO partner of KO-WT pairs (Fig. IB–E). Finally, reflecting the specificity of delivery to the pulmonary but not systemic vascular bed, there was no significant increase of miR-210 expression in endothelial cells isolated from aorta of KO partners of KO-WT pairs (Fig. IIF).
In response of delivery of miR-210 to pulmonary endothelium, although there was no significant mRNA change of miR-210-targets Iscu18 or Cox1029 in WT mice or in KO partners of WT-KO pairs (Fig. IIIA), the protein levels of ISCU1/2 (Fig. 2E) and COX10 (Fig. IIIB, IIID) in these miR-210-positive cells were reciprocally down-regulated as assessed by immunofluorescence, consistent with our prior report of predominant translational repression of ISCU1/2 by endothelial miR-210 in vivo17. Furthermore, consistent with prior reports of the control of TGF-β-SMAD signaling by miR-21030, decreased Smad3 was observed by miR-210 delivery (Fig. IIIC). Thus, by demonstrating the active functionality of this delivered miRNA, these parabiosis findings indicate that active and endogenous blood-borne miR-210 can be delivered chronically to the pulmonary vascular endothelium in vivo.
Specific pathogenic plasma factors were not altered in the context of hypoxic blood-borne miR-210 delivery.
We wanted to determine whether certain key soluble plasma-based factors relevant to PH pathogenesis could have been induced and transferred from one parabiont mouse to the other and thus influenced the effects of miR-210 delivery to PAECs. Namely, when comparing KO mice of KO-WT pairs with KO-KO pairs, there was no significant difference of vasoactive factors known to be important in PH pathogenesis, including endothelin-1 content in plasma (Fig. 2F) and nitrate/nitrite content in lungs indicative of nitric oxide release and metabolism (Fig. 2G). Furthermore, circulating levels of the inflammatory cytokine interleukin-6 (IL-6), a known driver of inflammation and PH31, were not altered (Fig. 2H). Thus, dysregulation of these specific vasoactive and inflammatory factors did not confound the actions of blood-borne miR-210 delivery.
PAEC delivery of blood-borne miR-210 promotes hemodynamic and histologic indices of PH.
Based on the endothelial uptake of extracellular miR-210, we wanted to determine if such delivery influenced the development of PH in vivo. In hypoxia, KO-KO parabiont pairs were protected from PH compared with WT-WT pairs, as displayed by lower right ventricular systolic pressure (RVSP) (Fig. 3A) and similar to prior measurements in isolated miR-210 KO mice17. Importantly, consistent with the increased delivery of pulmonary arterial endothelial miR-210, the KO mice in KO-WT pairs developed higher RVSP versus KO-KO pairs (Fig. 3A). As a reflection of right ventricular remodeling, right ventricular mass/body weight ratio was found to be increased in the KO partners in KO-WT pairs versus KO-KO pairs (Fig. 3B), consistent with alterations of RVSP in those same subjects. In situ, increased pulmonary arteriolar remodeling and muscularization were observed in the KO mice of KO-WT pairs versus KO-KO pairs and comparable to that seen in WT partners and WT-WT pairs (Fig. 3C). Thus, taken together, we observed pathogenic transport of active miR-210 to pulmonary endothelium in KO mice which consequently displayed aggravated hemodynamic and histopathologic indices of PH in vivo.
Figure 3. Delivery of blood-borne miR-210 promotes PH.
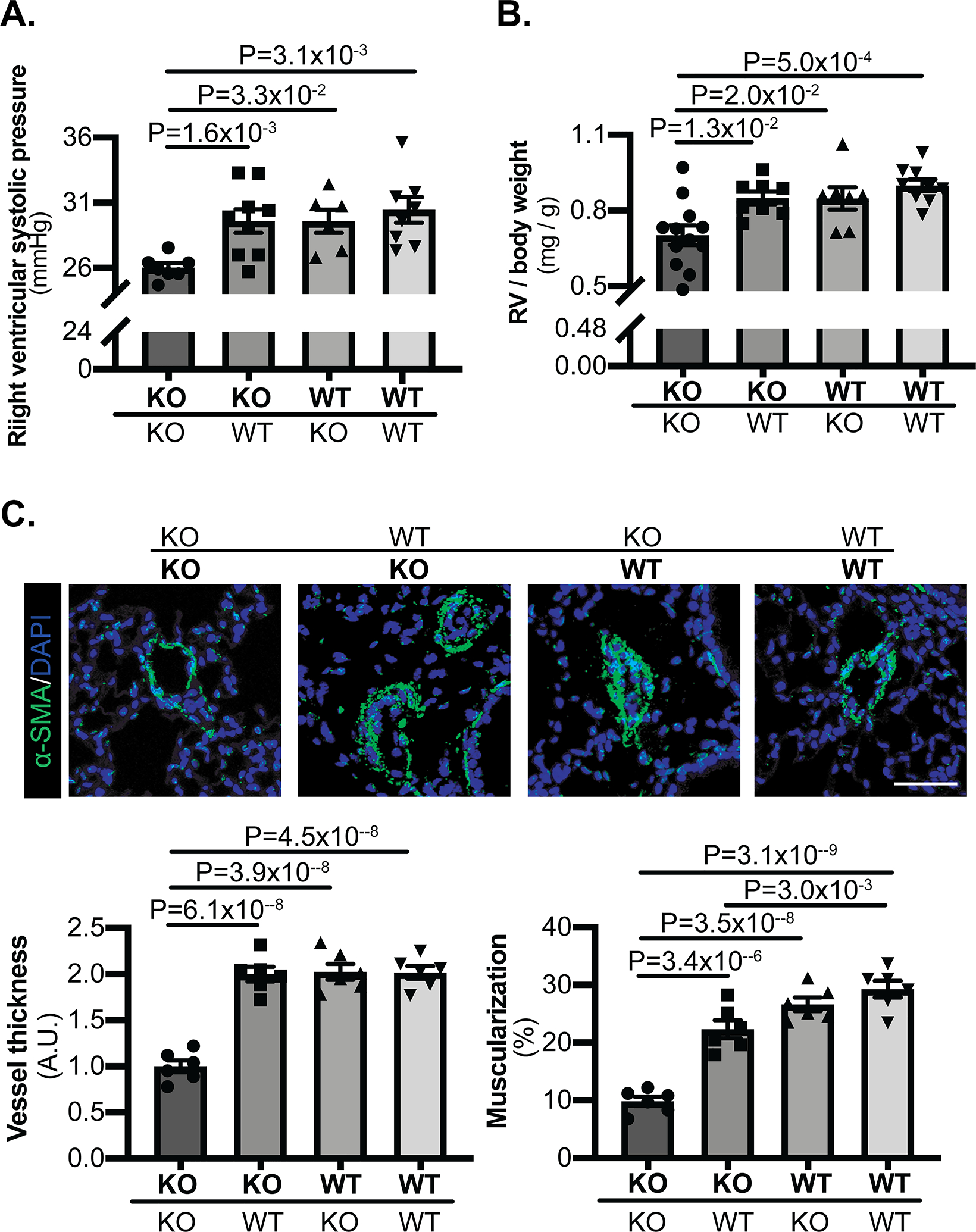
(A) By invasive right heart catheterization and measurement of right ventricular systolic pressure (RVSP), while hypoxic KO-KO pairs were protected from PH as compared with WT-WT pairs, the KO partners in KO-WT pairs developed significantly higher RVSP versus KO-KO pairs (N=7,9,6,8 mice). (B) Right ventricular mass/body weight ratio was increased in the KO partners of KO-WT pairs as compared with KO-KO pairs (N=12,8,7,9 mice). (C) In situ staining of α-SMA (green) demonstrated increased pulmonary arteriolar remodeling and muscularization in the KO partners of KO-WT pairs versus KO-KO pairs and comparable to that seen in WT partners and WT-WT pairs (N=6 mice/group, average of 10–12 vessels/mice. Scale bar denotes 50 μm.). (Data are presented as mean ± SEM. In x-axes of (A,B,C), bold font denotes parabionts being studied, whereas regular font denotes partner parabionts. One-way ANOVA with post-hoc Bonferroni testing was performed.)
Pulmonary vascular infiltration and expansion of miR-210-positive interstitial lung macrophages in vivo.
While our findings thus far suggested the importance of endocrine transport of miR-210 from blood to endothelial cells, paracrine transport was still possible, as our data only ruled out a role for paracrine mechanisms across vascular cell types (i.e., from smooth muscle cells, etc.). We also wanted to determine whether miR-210-expressing blood-borne cells may preferentially infiltrate the pulmonary vasculature and thus could initiate paracrine transport to the endothelium.
Utilizing the same parabiosis system and as assessed by fluorescence activated cell sorting (FACS) of isolated cells from blood and whole lung, we found increased presence of total blood monocytes, inflammatory Ly6chigh monocytes, and Ly6clow monocytes (Fig. 4A, IVA) in hypoxic WT-WT pairs as compared with hypoxic KO-KO pairs. Consistent with our prior findings that inflammatory Ly6chigh monocytes are the source of activated interstitial macrophages in hypoxic PH23, we found increased CD64/CD11b-positive interstitial lung macrophages (Fig. 4B–C, IVB) in the same hypoxic WT-WT pairs vs. KO-KO pairs, indicating increased mobilization of these specific cells. In that setting, a selective expansion of total and inflammatory Ly6chigh monocytes (Fig. 4A) as well as interstitial lung macrophages (Fig. 4B–C, IVB) was observed in KO mice of KO-WT pairs versus KO-KO partners. In situ staining of CD68 and miR-210 in mouse lung demonstrated the increased expansion of interstitial lung macrophages and arteriolar presence of miR-210-positive interstitial lung macrophages in KO mice of KO-WT pairs versus KO-KO partners (Fig. 4D). The presence of miR-210 in those FACS sorted interstitial lung macrophages in KO partners of KO-WT pairs was also confirmed by RT-qPCR (Fig. IVC). Thus, the inflammatory myeloid expansion of miR-210-expressing interstitial lung macrophages could serve as a paracrine source for endothelial delivery.
Figure 4. Hypoxic expansion of miR-210-positive blood monocytes and interstitial lung macrophages.
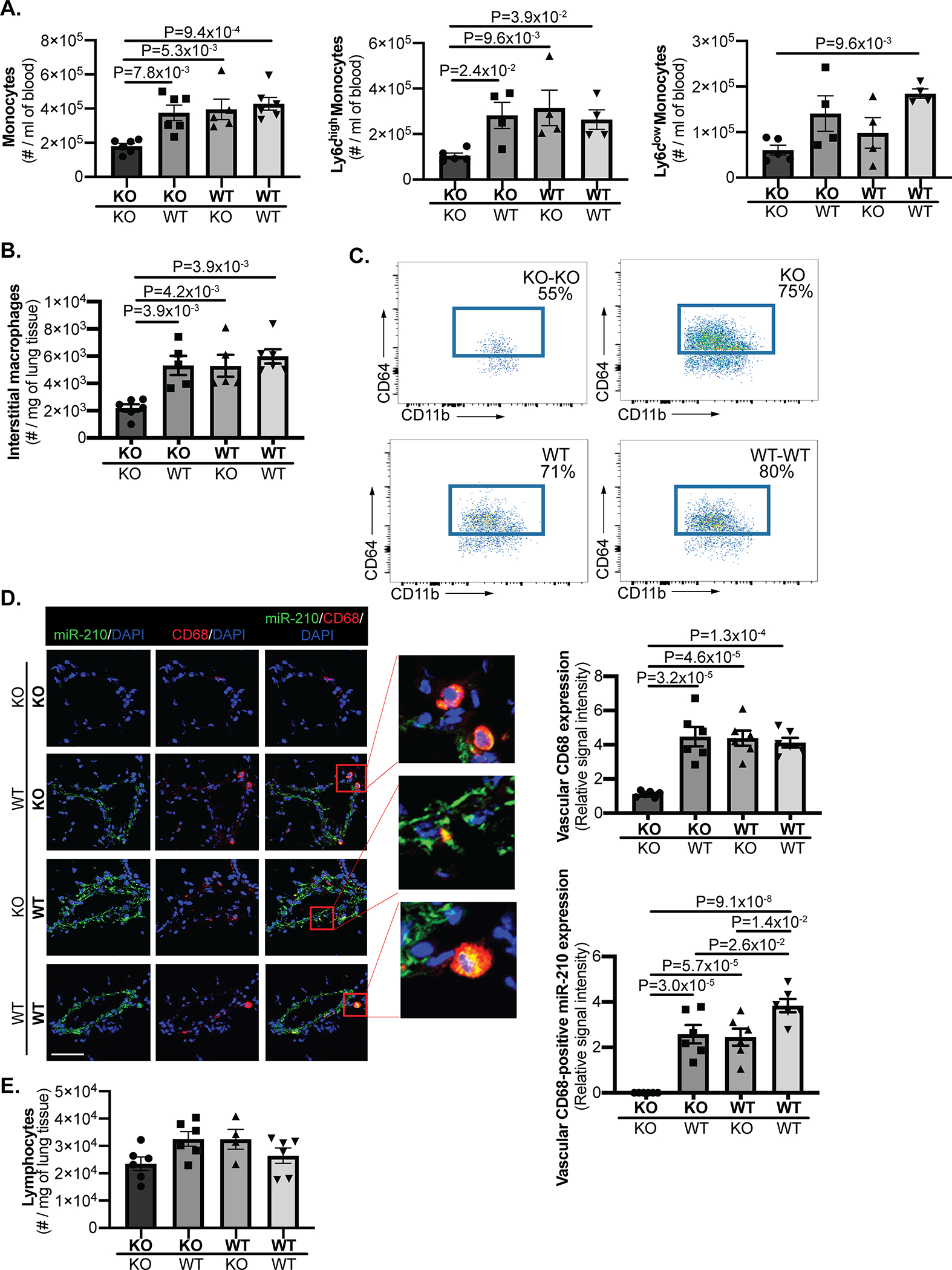
(A) Employing the same parabiosis system as in Figure 2 and via fluorescence activated cell sorting (FACS) of blood, total blood monocytes (N=6,6,5,6 mice), and subpopulations including Ly6chigh and Ly6clow monocytes (N=5,4,4,4 mice) were increased in hypoxic WT-WT pairs as compared with hypoxic KO-KO pairs. Importantly, KO mice of KO-WT pairs displayed an equivalent level of total blood monocytes and Ly6chigh monocytes, which was significantly higher than KO-KO pairs. (B-C) In parallel, by FACS, expansion of CD64/CD11b-positive interstitial lung macrophages was observed in isolated cells from whole lung of KO mice of KO-WT pairs comparable to that seen in the WT partner and WT-WT pairs but substantially increased compared with KO-KO pairs (quantitation in B (N=6,6,5,6 mice, and representative flow cytometric plots in C. Percentages reflect the frequencies of interstitial macrophages among CD45.2+ Siglec F- Ly-6G- CD11b+ MHC class II- leukocytes in the lung.). (D) Increased presence of miR-210-positive interstitial lung macrophages (yellow, micrograph inset) was also demonstrated by in situ staining of CD68 (red) and miR-210 (green) in the small pulmonary arterioles of KO mice of KO-WT pairs, comparable to WT partners and WT-WT pairs but greater in number than those seen in KO-KO pairs (N=6 mice/group, average of 10–12 vessels/mice. Scale bar denotes 50 μm.). (E) By FACS, no significant difference of lymphocyte presence was observed in lung tissue of KO-KO pairs, KO-WT pairs and WT-WT pairs (N=6,6,4,6 mice). (Data are presented as mean ± SEM. In x-axes of (A,B,D,E), bold font denotes parabionts being studied, whereas regular font denotes partner parabionts. One-way ANOVA with post-hoc Bonferroni testing was performed.)
To address whether miR-210 in ECs may be derived from hematopoietic cells earlier in myeloid commitment, we compared miR-210 levels between all hematopoietic stem and progenitor cells (HSPC) and myeloid cells in hypoxic mice. We found that miR-210 levels were 10-fold lower in HSPCs as compared with more differentiated myeloid cells (Fig. VA). Furthermore, in lung tissue of those mice, frequencies of HSPC, myeloid progenitors (CMP), lymphoid progenitors (CLP), and granulocyte-monocyte progenitors (GMP) were exceptionally low, quantified between 0–1.8% of the total lung leukocyte population (Fig. VB-C) and thus could not be robust sources of miR-210. To investigate if miR-210 could influence the proliferation or expansion of myeloid precursors, we found that forced expression of miR-210 in cultured HSPCs from normoxic mice did not affect lineage commitment (Fig. VD-E). Thus, taken together, these data argue against the notion that these progenitor cells are the source of delivered miR-210 throughout the pulmonary endothelium or the underlying reason for worsening PH.
Finally, beyond cells of myeloid lineage, while pathogenic lymphocyte engraftment and activity in the pulmonary vasculature of certain subtypes of PH have been reported32, we found no difference in lung lymphocyte content of hypoxic WT-WT pairs vs. KO-KO pairs (Fig. 4E). Nonetheless, the presence of significant lymphocytes in lung tissue could in theory serve as a paracrine source of miR-210. For other hematologic populations that could potentially engraft near the pulmonary vasculature, we performed immunofluorescence staining in the lung for cellular markers including NK1.1 for NK cells, CD11c for dendritic cells, CD41 for platelets and basophils, and Siglec-F for eosinophils. These cell populations encompassed an exceedingly rare minority in the hypoxic lung and, when present, were typically outside the vasculature (Fig. VIA). Thus, these cells would not serve as robust sources of miR-210. In sum, we found only myeloid and lymphoid cells to represent hematologic populations that could possibly engraft near the lung vasculature in high enough numbers to account for the observed delivery of miR-210 to lung endothelium.
PAEC delivery and pathogenic activity of blood-borne miR-210 does not depend on vascular engraftment and paracrine delivery by myeloid cells or lymphoid cells.
To differentiate the pathogenic contributions of cell-free endocrine vs. myeloid-dependent or lymphoid-dependent paracrine delivery of blood-borne miR-210, parabiosis pairs were devised to ablate specifically myeloid or lymphoid engraftment to lung vasculature. Namely, Cx3cr1 is a chemokine receptor gene expressed predominantly on myeloid cells and principally controls the recruitment of myeloid cells from bone marrow to lung vasculature in PH23. Rag1 activates immunoglobulin recombination, and its genetic deletion results in a well-established mouse model lacking all mature lymphocytes33. As such, we studied miR-210 KO-WT parabiosis where the miR-210 WT parabiont was with or without deficiency in Cx3cr1 or Rag1 (Fig. 5A). Specifically, (miR-210 WT; Cx3cr1 KO) mice would be unable to mobilize and transfer miR-210-positive myeloid cells to the miR-210 KO parabiont partner. Similarly, (miR-210 WT; Rag1 KO) mice would be unable to transfer miR-210-positive lymphocytes to the miR-210 KO parabiont partner.
Figure 5. Transfer of miR-210-positive myeloid cells and lymphocytes was abrogated by Cx3cr1 deficiency and Rag1 deficiency, respectively.
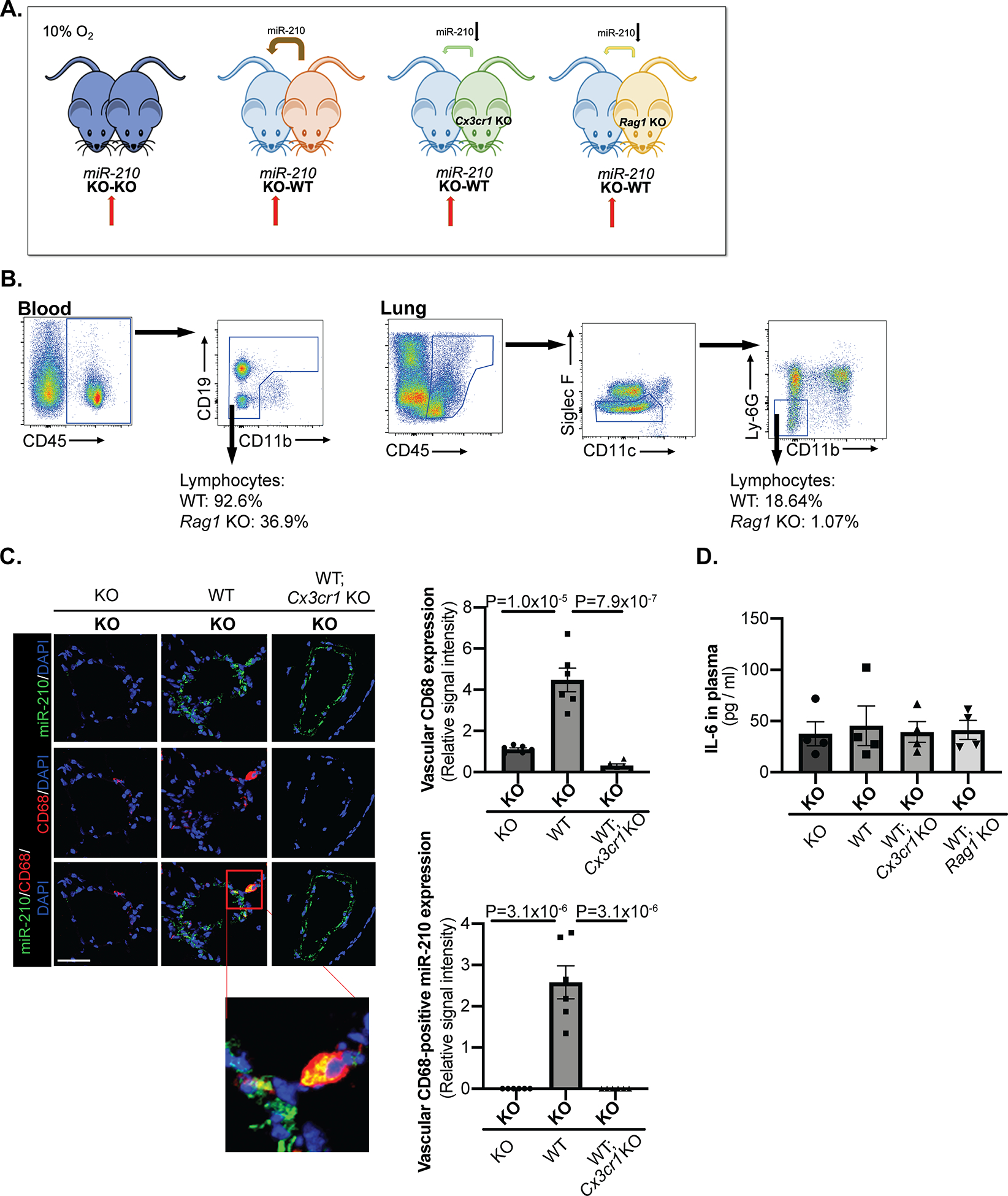
(A) A parabiosis platform similar to that in Figure 2 was used in the context of pairing miR-210 −/− (KO) mice with miR-210 −/− (KO), miR-210 +/+ (WT), miR-210 +/+; Cx3cr1 −/− (WT; Cx3cr1 KO) or miR-210 +/+; Rag1 −/− (WT; Rag1 KO) mice. One week after surgery, the mouse pairs were exposed to chronic hypoxia (10% O2) for three weeks. (Red arrows denote comparison groups) (B) As denoted by frequencies (%) of enriched lymphocytes within the total leukocyte population, lack of mature lymphocytes was confirmed by flow cytometry in blood and lung tissue of (WT; Rag1 KO mice). (C) Decreased presence of miR-210-positive interstitial lung macrophages (yellow, micrograph inset) was demonstrated by in situ staining of CD68 (red) and miR-210 (green) in the small pulmonary arterioles of KO partners of KO-(WT; Cx3cr1 KO) pairs, comparable to those seen in KO-KO pairs (N=6 mice/group, average of 10–12 vessels/mice. Scale bar denotes 50 μm.). (D) In parallel, circulating levels of inflammatory marker IL6 were not altered in plasma of KO partners of KO-WT pairs, KO-(WT; Cx3cr1 KO) pairs and KO-(WT; Rag1 KO) compared to KO-KO pairs, as quantified by ELISA (N=4 mice/group). (Data are presented as mean ± SEM. In x-axes of (C,D), bold font denotes parabionts being studied, whereas regular font denotes partner parabionts. Kruskal Wallis and post-hoc Dunn’s testing for D and one-way ANOVA with post-hoc Bonferroni testing for other panels were performed.)
Consistent with published literature33, lymphocyte deficiency in blood and particularly in the lung of Rag1 KO mice was demonstrated by flow cytometry (Fig. 5B), thus assuring negligible miR-210-positive lymphocyte engraftment into lung tissue. Unlike Rag1 control over mature lymphocytes, however, Cx3cr1 knockout does not entirely ablate the myeloid cell lineage. Thus, in Cx3cr1 KO parabiosis pairs, we aimed to quantify monocyte and macrophage populations in relation to miR-210 expression. After 3 weeks in hypoxia, KO partners of KO – (miR-210 WT; Cx3cr1 KO) pairs displayed decreased number of total monocytes in blood (Fig. IVD) and decreased number of CD64/CD11b-positive interstitial macrophages in lung by FACS (Fig. IVE) which were comparable to that seen in KO-KO pairs. Attenuated interstitial lung macrophage expansion and near complete abrogation of the presence of miR-210-positive interstitial lung macrophages were confirmed by in situ staining of CD68 and miR-210 in the small pulmonary arterioles of KO partners of KO – (miR-210 WT; Cx3cr1 KO) pairs, again comparable to those seen in KO-KO pairs (Fig. 5C). These findings of near negligible miR-210 expression in isolated CD64/CD11b-positive interstitial lung macrophages were also observed by RT-qPCR (Fig. IVF). As with KO-WT pairs described previously, circulating inflammatory IL-6 in plasma was not altered in KO partners paired with (miR-210 WT; Cx3cr1 KO) mice or (miR-210 WT; Rag1 KO) as compared with KO-KO pairs (Fig. 5D).
Despite abrogating inflammatory myeloid and lymphoid engraftment and any putative paracrine transport of miR-210 from these cells (Fig. 5B–C, IVD–F), presence of miR-210 in plasma (Fig. 6A) and in CD31+/CD45− lung endothelial cells (Fig. 6B) of KO partners of KO – (miR-210 WT; Cx3cr1 KO) and of KO – (miR-210 WT; Rag1 KO) pairs still displayed similar levels of up-regulation as when paired with WT mice. In situ, corresponding and equivalent decrease of ISCU1/2 (Fig. 6C) and COX10 (Fig. IIID) proteins was observed in KO partners of KO-WT, KO – (miR-210 WT; Cx3cr1 KO), and KO – (miR-210 WT; Rag1 KO) pairs. Consequently, RVSP (Fig. 6D), right ventricular remodeling (Fig. 6E), and pulmonary arteriolar remodeling and muscularization (Fig. 6F) displayed comparable elevation across all KO parabionts of KO-WT, KO – (miR-210 WT; Cx3cr1 KO) and KO – (miR-210 WT; Rag1 KO) pairs. Thus, taken together, endogenous delivery and PH pathogenic activity of blood-borne miR-210 do not rely upon hematologic blood cell engraftment but rather on extracellular, circulating forms of miR-210 for long distance, endocrine signaling (Fig. 7).
Figure 6. PAEC delivery and pathogenic activity of blood-borne miR-210 depends upon extracellular endocrine, rather than myeloid-based or lymphoid-based paracrine, delivery.
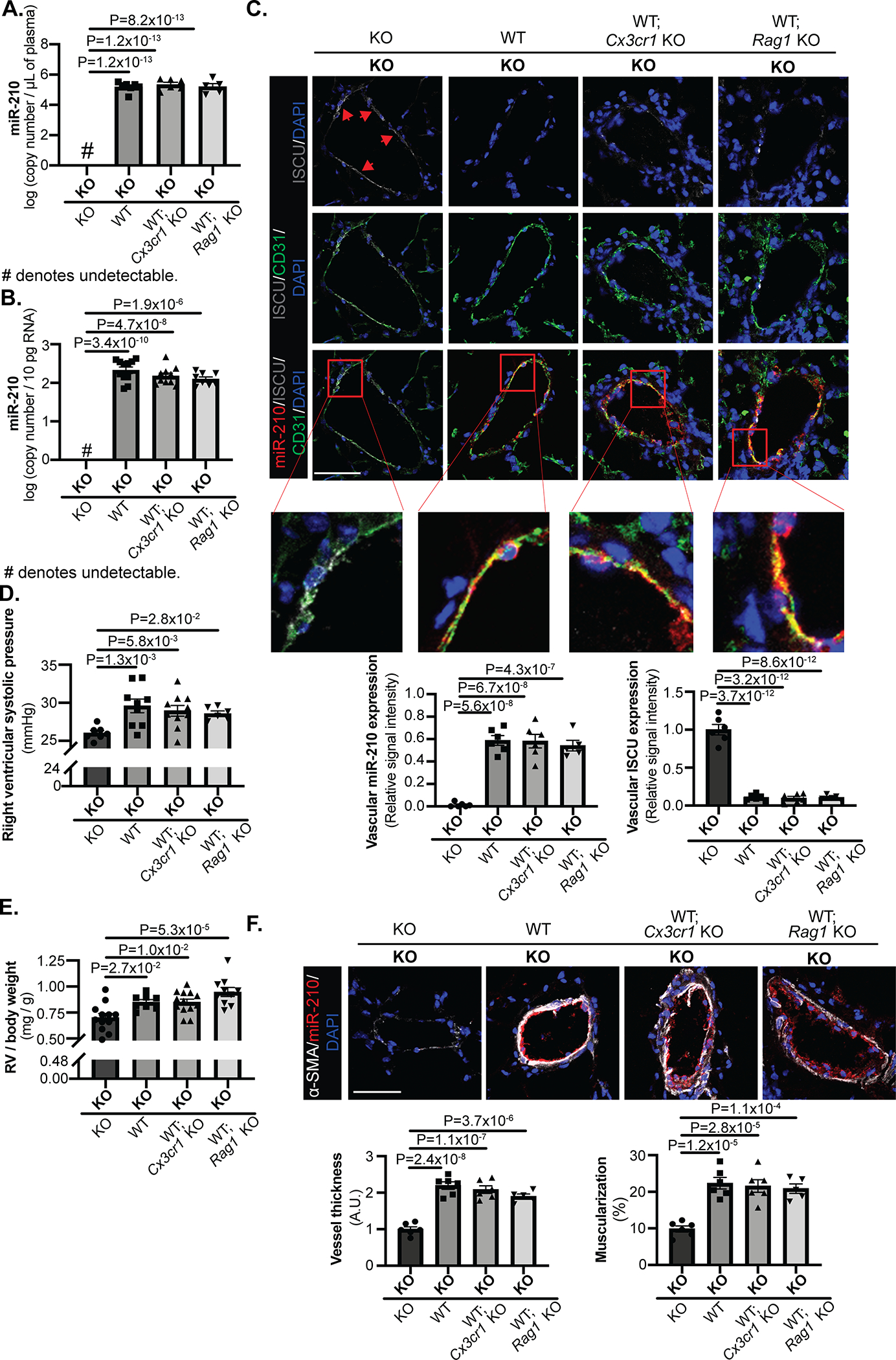
(A-B) Mouse parabiosis pairs as in Figure 5 were exposed to chronic hypoxia (10% O2) for 3 weeks to induce endogenous miR-210 up-regulation. Levels of miR-210 in plasma (A, N=8,7,6,5 mice) and CD31+/CD45− lung endothelial cells (B, N=11,11,10,8 mice) were assessed by RT-qPCR. In KO partners of KO-(WT; Cx3cr1 KO) pairs and KO-(WT; Rag1 KO pairs), they displayed similar extracellular (A) and intracellular (B) miR-210 levels as in KO partners of KO-WT pairs, which was substantially higher than expression in KO-KO pairs. (C) By in situ staining of miR-210 (red), its target proteins ISCU1/2 (grey, pointed out by red arrows), and the endothelial marker CD31 (green), comparable level of miR-210 delivery to pulmonary arteriolar endothelium (yellow, micrograph inset) and corresponding decrease of ISCU1/2 in the KO partners of KO-(WT; Cx3cr1 KO) pairs and of KO-(WT; Rag1 KO) pairs were detected as compared with KO partners of KO-WT pairs (N=6,6,6,5 mice, average of 10–12 vessels/mice. Scale bar denotes 50 μm.). (D-F) In the aspect of pathogenic phenotypes, right ventricular systolic pressure (RVSP) (D, N=7,9,10,6 mice), right ventricular remodeling (E, N=12,8,13,10 mice) and pulmonary arteriolar remodeling and muscularization (F, N=6,6,6,5 mice, average of 10–12 vessels/mice. Scale bar denotes 50 μm.) also showed comparable degree of elevation in KO partners of KO-(WT; Cx3cr1 KO) pairs and of KO-(WT; Rag1 KO) pairs as KO partners of KO-WT pairs, measured by right heart catheterization, RV mass to body weight ratio and in situ staining of α-SMA (white), respectively. (Data are presented as mean ± SEM. In all panels, bold font in x-axes denotes parabionts being studied, whereas regular font denotes partner parabionts. One-way ANOVA with post-hoc Bonferroni testing was performed.)
Figure 7. Model of the endocrine delivery and pathogenic actions of blood-borne miR-210 in PH.
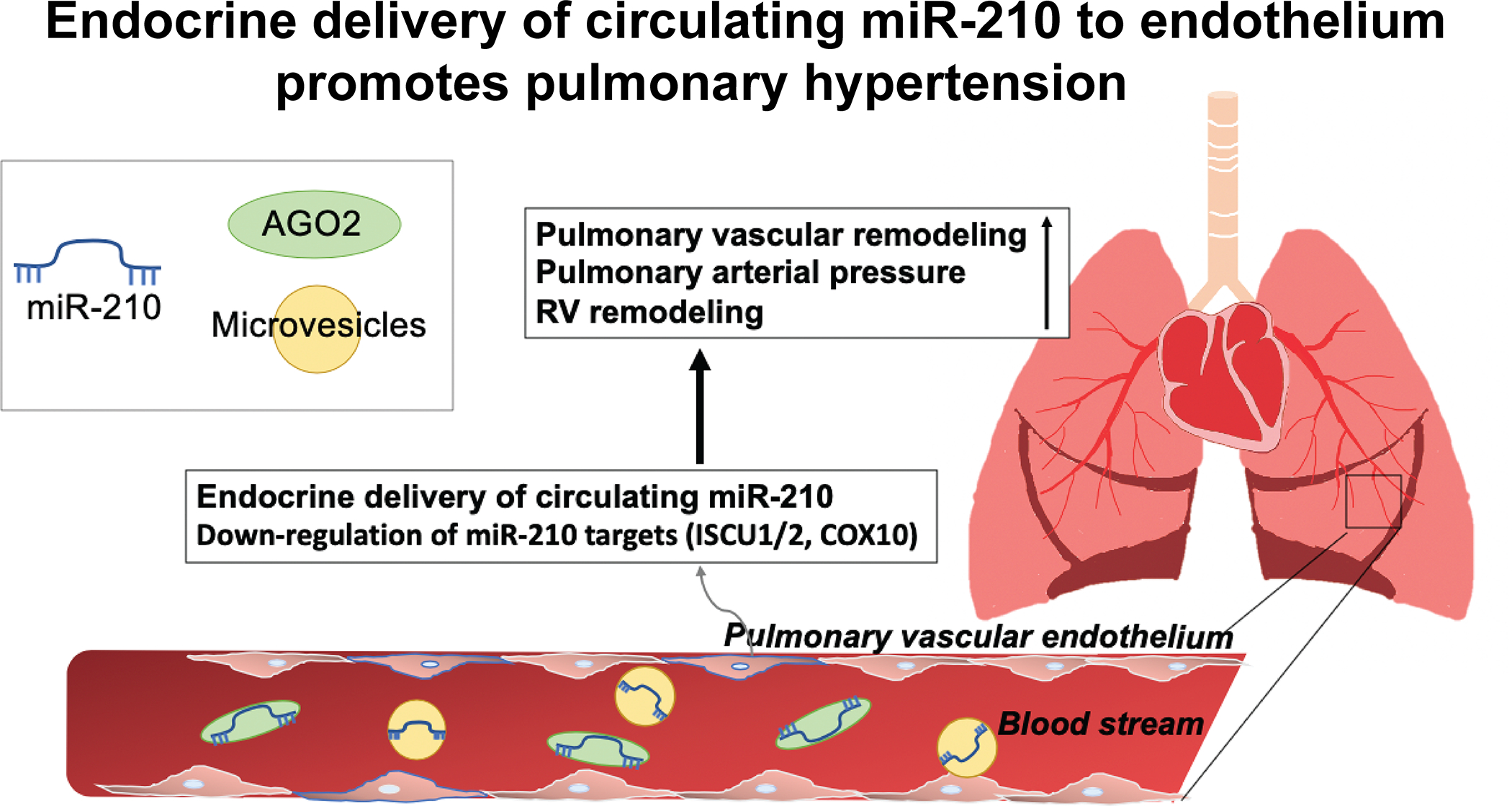
Collectively, our findings demonstrate that long range, endocrine transport encompasses the predominant mechanism by which blood-borne miR-210 is delivered into pulmonary arterial endothelial cells with consequent control of PH in vivo.
DISCUSSION
Based on use of BMT and chronic parabiosis discovery platforms in vivo, we conclude that long-range extracellular transport of miR-210, with consequent uptake into pulmonary vascular endothelial cells, promotes PH (Fig. 7). These findings offer long-awaited evidence of endocrine and disease-modifying activities of extracellular miRNAs, thus expanding our fundamental understanding of the systemic physiology of miRNA activity in the vasculature and beyond. They also define the multi-level, RNA-mediated system of anatomic crosstalk beyond the cardiopulmonary space of hypoxic signaling and PH. These results carry a number of implications not only for expanding our understanding of PH pathogenesis in general but also for potential extrapolation to other human diseases (such as cancer, neurologic disease, cardiomyopathies) where hypoxic injury and metabolic dysregulation are present. Finally, these findings provide a translational imperative for developing blood-based miR-210 technologies for diagnosis and therapy in this complex disease.
As a result of such direct proof of endocrine actions of miR-210 in the diseased pulmonary vasculature, there exist fundamental implications for understanding non-coding RNA biology both within and beyond the vasculature. While a prior study had used parabiosis to study the long-range transport of extracellular forms of the miRNA cluster miR-25–93-106b to control obesity11, that proof was confounded by transfer of leptin and adiponectin that carried significant effects on adipocyte function. Additionally, that study did not rule out all possible hematologic cell sources of paracrine delivery. Thus, our data are the first to offer direct evidence of long-range, chronic transfer between anatomically distinct tissues. Consequently, our findings substantially allay skepticism regarding the perceived stoichiometric challenges of endocrine miRNA delivery. Namely, given the low levels of expression of circulating miRNAs in plasma as compared with baseline endogenous intracellular levels in most cell types, unfavorable gradients and rate of miRNA delivery from the bloodstream in general have been cited as substantial barriers to achieving meaningful intracellular levels by miRNA transport alone1, 12. At the molecular level, those challenges could be alleviated by specialized and dynamic uptake mechanisms, such as the hypoxia-dependent prolyl-hydroxylation-dependent switch on AGO2 that we found previously controls endothelial miR-210 import in cultured PAECs19. It is possible that such a switch may figure prominently in assuring more efficiency of miR-210 endocrine transfer. Alternatively, successful endocrine transfer may primarily depend upon the chronicity of exposure across time to extracellular miRNAs, leading to a putative slow but steady stockpiling of delivered miRNAs in recipient cells. If so, it is likely that such slow kinetics would be affected by intracellular miRNA degradation pathways as well, which would factor greatly into the final steady state conditions. Future time-course studies for miR-210 will be important to support this notion.
Moreover, while our data define the endocrine nature of miR-210, the exact contributions of extracellular miRNA packaging to endocrine transport await characterization. Specifically, we have reported that circulating and active miR-210 can be packaged either in microparticles or RNA-protein or RNA-lipoprotein complexes19 for transfer to cultured PAECs. Future studies are warranted to determine: (A) whether and how specific packaging of miR-210 or other miRNAs can control overall endocrine rates of transport and (B) whether such packaging is dictated by the bone marrow vs. non-bone marrow derived sources of plasma miR-210 (Fig. 1). Furthermore, while our findings emphasize an endocrine function for miR-210, our findings may not be relevant to all miRNAs. Because of the low baseline expression of miR-210 in PAECs, transport of miR-210 specifically may be particularly amenable for biological activity. It remains to be seen if other circulating miRNAs can also be delivered to the pulmonary vasculature in meaningful concentrations, particularly if baseline endogenous vascular expression in recipient cells is already at a high level. In those cases, paracrine transport of closely aligned cells may be more relevant13, as suggested by prior delivery studies relying upon supraphysiologic extracellular levels of miRNAs in vitro and in vivo5, 8, 9, 13. Finally, other non-coding RNAs beyond miRNAs may engage in similar or simultaneous long-distance transport. If so, particularly for non-coding RNAs that tend to act distinctly from miRNAs, an additional variable to decipher is the efficiency of subcellular transport to the nucleus or other organelles beyond that observed with miR-210 delivery19.
Our observations also engender a number of intriguing models to explain the RNA-controlled specificity of cell-cell communication. First, while our data of endocrine transfer from blood to vasculature focuses attention on the endothelium, we also do not rule out the possibility of paracrine or endocrine transport to smooth muscle or adventitia outside of the boundaries of this system. Second, we found miR-210 delivery to be unique to the pulmonary, as compared to the systemic, vascular endothelium. Although the mechanism driving this vascular bed specificity is unclear, there is a long-standing appreciation of the functional differences of endothelium from the pulmonary and systemic circulations that could underlie this observation. More specifically, differences in how pulmonary vs. systemic vessel endothelial cells respond to hypoxia have been reported34, and this may align with our prior findings of differences of how delivered miR-210 is activated in hypoxia vs. reoxygenation19. Alternatively, biomechanical stressors to the endothelium of both blood flow and respiratory motion may alter the cellular susceptibility for delivery. Notably, however, for any such mechanism, the physiologic distinctions of pulmonary vascular endothelium would have to be consistent across both arteries and veins in the lungs (Fig. IID). Third, we found that while both the passenger strand miR-210–5p and precursor miR-210 molecules were transported in blood, they were not detectable in lung endothelium (Fig. IB–E). These data may reflect an additional layer of miRNA strand specificity to such transport and delivery. Alternatively, because our platform did not allow for tracking of individual molecules, we cannot rule out the possibility of endothelial delivery followed by rapid and preferential degradation in relation to the mature miR-210 strand28. Fourth, while our results demonstrated that the infiltration and expansion of miR-210-positive interstitial lung macrophages or lymphocytes were not essential for miR-210 delivery and PH, our findings do not rule out a contributing role. Finally, it is unknown whether and how bidirectional endocrine transport may occur in certain contexts from the vasculature to the bone marrow and beyond.
In the context of the biology of hypoxia, endocrine transport of active circulating miR-210 may have further implications in multiple physiologic contexts in cardiovascular disease and health. Notably, these findings may have relevance in remote ischemic preconditioning (remote IPC), a protocol used clinically in humans to reduce infarct size in the myocardium or other organs35. Remote IPC may rely upon the uptake of factors directly regulated by the master transcription factor of hypoxia, HIF-1α36, including novel metabolites such as kynurenic acid37. However, the full complement of such HIF-dependent molecular effectors remains undefined. The functions of a transported and delivered HIF-inducible miR-210 mirror key observations found in remote IPC38–40 in mouse models of peripheral arterial disease41 and myocardial42 ischemia – namely, repression of mitochondrial respiration. The therapeutic benefit of miR-210 in fibrotic caps of carotid arteries also may suggest a role for this extracellular miRNA in atherosclerotic cell-cell communication43. Future work to link more definitively circulating miR-210 to remote IPC and beyond could have broad implications for many diseases, including cancer and neurologic diseases among others, where hypoxic injury and metabolic dysregulation are present.
In regard to PH, our results delineate a pathogenic, RNA-based, endocrine mechanism that contributes substantially to the already known complex behavior of miRNAs in this disease. A growing number of miRNAs are known to be dysregulated in diseased pulmonary vascular cells with pervasive and pleiotropic regulatory functions1. The fact that blood-borne miR-210 plays a causative role in PH pathogenesis furthers the need to develop a systems-wide consideration of miRNAs that span beyond cardiopulmonary tissue. Although we demonstrated that bone marrow contributes substantially to plasma miR-210, the entire complement of tissue sources outside the cardiopulmonary compartment that releases miR-210 as well as any potential alterations of their communication kinetics with the pulmonary vasculature in PH, remains incompletely defined (Fig. 1). It also remains unclear whether delivery of miR-210 in vivo may allow for a heightened state of target gene engagement based on AGO2 activation, as our in vitro analyses have suggested19. It is possible that true insight into RNA-specific pathogenic processes in PAECs may demand the development of a strategy to differentiate delivered, and putatively more active, miR-210 molecules from the endogenously produced, and potentially less active, miR-210 population in recipient cells.
These new pathobiologic notions of circulating miR-210 in PH also offer support for clinical strategies aimed at tracking and modulating this miRNA in anatomically more accessible blood and plasma. Previous studies have reported dysregulation of PH-relevant circulating miRNA levels in human patients5. Our mechanistic findings here justify more substantive efforts to pursue detailed and quantitative analyses of miR-210 expression over time in various PH subtypes for diagnostic and prognostic opportunity. Perhaps more importantly, our findings indicate the possibility that specific inhibition of miR-210 in the blood could be an effective strategy to prevent or treat PH. Such an approach would obviate the need to target miRNA therapies directly to the pulmonary vasculature and thus circumvent the historically challenging issue of tissue specificity of delivery and legitimate worry of non-specific effects of miRNA therapies when delivered systemically.
Limitations to this study and opportunities for future investigation should be acknowledged. Here, we utilized a chronic hypoxia model of PH in mice which does not fully recapitulate all aspects of human pulmonary arterial hypertension and may be more reflective of WSPH Group 3 PH due to hypoxic lung disease. Future work with parabiosis of other models of PH will be important to determine if blood-borne miR-210 is similarly crucial for control over disease manifestation. Future studies should also be designed to determine whether pathogenic extracellular miR-210 is primarily delivered to PAECs in the form of microvesicles, RNA-protein complexes, or both.
In sum, we demonstrate that long range, endocrine transport of blood-borne miR-210 into pulmonary vascular endothelial cells promotes PH in vivo. These findings broaden our fundamental understanding of miRNA physiology at the organ system level. Moreover, this work defines a multi-faceted system of RNA-based crosstalk in PH, providing a basis for developing blood-based miR-210 technologies for diagnosis, prognosis, and therapy in this complex and fatal disease.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Extracellular microRNAs (miRNAs) circulating in the blood stream are dynamically regulated in human health and disease, including a deadly vascular and lung condition called pulmonary hypertension (PH).
The long-range tissue uptake and endocrine activity of extracellular blood-borne miRNAs are incompletely understood.
Proof of long-range endocrine delivery of these molecules has been elusive and confounded by multiple variables.
What New Information Does This Article Contribute?
A majority of hypoxia-induced microRNA-210 (miR-210) circulating in the blood during systemic hypoxic exposure originates from the bone marrow.
During chronic hypoxic exposure, miR-210 in the blood is transported into the endothelial lining of blood vessels of the lung promoting PH.
For more than a decade, extracellular miRNAs in the blood have been studied as biomarkers and effectors of human health and disease. Their long-range tissue uptake and potential endocrine activity across anatomic space is incompletely understood. In PH, a deadly condition of the lung blood vessels, the hypoxia-inducible miR-210 in pulmonary endothelial cells promotes disease, but its activity as an extracellular molecule has been incompletely defined. We sought to determine whether endogenous endocrine delivery of extracellular miR-210 to pulmonary vascular endothelial cells promotes PH. Using miR-210 replete and knockout mice, we tracked blood-borne miR-210 using bone marrow transplantation and parabiosis (conjoining of circulatory systems). We found that circulating miR-210 was derived predominantly from bone marrow. Via parabiosis, blood-borne miR-210 in plasma was delivered to lung endothelium, accompanied by down-regulation of miR-210 target genes and PH development. The findings of endogenous blood-borne transport of miR-210 and RNA-mediated crosstalk across organ systems adds to our understanding of the systemic role of extracellular miRNA activity.
SOURCES OF FUNDING
This work was supported by NIH grants R01 HL124021, HL122596, HL 138437, and UH2/UH3 TR002073 as well as AHA grants 14GRNT19600012 and 18EIA33900027 (SYC) and supported by French National Research Agency ANR-18-CE14–0025 (TB).
Nonstandard Abbreviations and Acronyms:
- AGO2
Argonaute 2
- BMT
Bone marrow transplant
- FACS
Fluorescence activated cell sorting
- HIF-1α
Hypoxia inducible factor-1 alpha
- Fe-S
Iron-sulfur
- ISCU1/2
Iron-sulfur assembly proteins
- IPC
Ischemic preconditioning
- KO
Knockout
- miRNA
microRNA
- miR-210
microRNA-210
- PAECs
Pulmonary arterial endothelial cells
- PAH
Pulmonary arterial hypertension
- PH
Pulmonary hypertension
- RT-qPCR
Reverse transcription-quantitative polymerase chain reaction
- RVSP
Right ventricular systolic pressure
- WT
Wild-type
- WSPH
World Symposium on Pulmonary Hypertension
Footnotes
DISCLOSURES
SYC has served as a consultant for Zogenix, Aerpio, and United Therapeutics; SYC is a director, officer, and shareholder in Numa Therapeutics; SYC holds research grants from Actelion and Pfizer. SYC and TB have filed patent applications regarding the targeting of metabolism in pulmonary hypertension. Other authors: none.
REFERENCES
- 1.Negi V, Chan SY. Discerning functional hierarchies of micrornas in pulmonary hypertension. JCI Insight. 2017;2:e91327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aliotta JM, Pereira M, Wen S, Dooner MS, Del Tatto M, Papa E, Goldberg LR, Baird GL, Ventetuolo CE, Quesenberry PJ, Klinger JR. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc Res. 2016;110:319–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Creemers EE, Tijsen AJ, Pinto YM. Circulating micrornas: Novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012;110:483–495 [DOI] [PubMed] [Google Scholar]
- 4.He Y, Lin J, Kong D, Huang M, Xu C, Kim TK, Etheridge A, Luo Y, Ding Y, Wang K. Current state of circulating micrornas as cancer biomarkers. Clin Chem. 2015;61:1138–1155 [DOI] [PubMed] [Google Scholar]
- 5.Min PK, Chan SY. The biology of circulating micrornas in cardiovascular disease. Eur J Clin Invest. 2015;45:860–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mrnas and micrornas is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659 [DOI] [PubMed] [Google Scholar]
- 7.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating micrornas independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–5008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung KO, Youn H, Lee CH, Kang KW, Chung JK. Visualization of exosome-mediated mir-210 transfer from hypoxic tumor cells. Oncotarget. 2017;8:9899–9910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig V, Tzankov A, Flori M, Schmid C, Bader A, Müller A. Systemic microrna-34a delivery induces apoptosis and abrogates growth of diffuse large b-cell lymphoma in vivo. Leukemia. 2012;26:2421. [DOI] [PubMed] [Google Scholar]
- 10.Turchinovich A, Weiz L, Burwinkel B. Extracellular mirnas: The mystery of their origin and function. Trends Biochem Sci. 2012;37:460–465 [DOI] [PubMed] [Google Scholar]
- 11.Cioffi M, Vallespinos-Serrano M, Trabulo SM, Fernandez-Marcos PJ, Firment AN, Vazquez BN, Vieira CR, Mulero F, Camara JA, Cronin UP. Mir-93 controls adiposity via inhibition of sirt7 and tbx3. Cell Rep. 2015;12:1594–1605 [DOI] [PubMed] [Google Scholar]
- 12.Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, Cheng HH, Arroyo JD, Meredith EK, Gallichotte EN. Quantitative and stoichiometric analysis of the microrna content of exosomes. Proc Nat Acad Sci. 2014;111:14888–14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng L, Blanco FJ, Stevens H, Lu R, Caudrillier A, McBride M, McClure JD, Grant J, Thomas M, Frid M, Stenmark K, White K, Seto AG, Morrell NW, Bradshaw AC, MacLean MR, Baker AH. Microrna-143 activation regulates smooth muscle and endothelial cell crosstalk in pulmonary arterial hypertension. Circ Res. 2015;117:870–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, Sun F, Lu J, Yin Y, Cai X, Sun Q, Wang K, Ba Y, Wang Q, Wang D, Yang J, Liu P, Xu T, Yan Q, Zhang J, Zen K, Zhang CY. Secreted monocytic mir-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133–144 [DOI] [PubMed] [Google Scholar]
- 15.Njock MS, Cheng HS, Dang LT, Nazari-Jahantigh M, Lau AC, Boudreau E, Roufaiel M, Cybulsky MI, Schober A, Fish JE. Endothelial cells suppress monocyte activation through secretion of extracellular vesicles containing antiinflammatory micrornas. Blood. 2015;125:3202–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang AL, Vita JA. Effects of systemic inflammation on endothelium-dependent vasodilation. Trends Cardiovasc Med. 2006;16:15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White K, Lu Y, Annis S, Hale AE, Chau BN, Dahlman JE, Hemann C, Opotowsky AR, Vargas SO, Rosas I, Perrella MA, Osorio JC, Haley KJ, Graham BB, Kumar R, Saggar R, Saggar R, Wallace WD, Ross DJ, Khan OF, Bader A, Gochuico BR, Matar M, Polach K, Johannessen NM, Prosser HM, Anderson DG, Langer R, Zweier JL, Bindoff LA, Systrom D, Waxman AB, Jin RC, Chan SY. Genetic and hypoxic alterations of the microrna-210-iscu1/2 axis promote iron-sulfur deficiency and pulmonary hypertension. EMBO Mol Med. 2015;7:695–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. Microrna-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins iscu1/2. Cell Metab. 2009;10:273–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hale A, Lee C, Annis S, Min PK, Pande R, Creager MA, Julian CG, Moore LG, Mitsialis SA, Hwang SJ, Kourembanas S, Chan SY. An argonaute 2 switch regulates circulating mir-210 to coordinate hypoxic adaptation across cells. Biochim Biophys Acta. 2014;1843:2528–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan L, Chen X, Talati M, Nunley BW, Gladson S, Blackwell T, Cogan J, Austin E, Wheeler F, Loyd J, West J, Hamid R. Bone marrow-derived cells contribute to the pathogenesis of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2016;193:898–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pugliese SC, Kumar S, Janssen WJ, Graham BB, Frid MG, Riddle SR, El Kasmi KC, Stenmark KR. A time- and compartment-specific activation of lung macrophages in hypoxic pulmonary hypertension. J Immunol. 2017;198:4802–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar R, Mickael C, Kassa B, Gebreab L, Robinson JC, Koyanagi DE, Sanders L, Barthel L, Meadows C, Fox D, Irwin D, Li M, McKeon BA, Riddle S, Dale Brown R, Morgan LE, Evans CM, Hernandez-Saavedra D, Bandeira A, Maloney JP, Bull TM, Janssen WJ, Stenmark KR, Tuder RM, Graham BB. Tgf-beta activation by bone marrow-derived thrombospondin-1 causes schistosoma- and hypoxia-induced pulmonary hypertension. Nat Commun 2017;8:15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Florentin J, Coppin E, Vasamsetti SB, Zhao J, Tai YY, Tang Y, Zhang Y, Watson A, Sembrat J, Rojas M, Vargas SO, Chan SY, Dutta P. Inflammatory macrophage expansion in pulmonary hypertension depends upon mobilization of blood-borne monocytes. J Immunol. 2018;200:3612–3625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang L, Ma Q, Li Y, Li B, Zhang L. Inhibition of microrna-210 suppresses pro-inflammatory response and reduces acute brain injury of ischemic stroke in mice. Exp Neurol. 2018;300:41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mok Y, Schwierzeck V, Thomas DC, Vigorito E, Rayner TF, Jarvis LB, Prosser HM, Bradley A, Withers DR, Martensson IL, Corcoran LM, Blenkiron C, Miska EA, Lyons PA, Smith KG. Mir-210 is induced by oct-2, regulates b cells, and inhibits autoantibody production. J Immunol. 2013;191:3037–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Flach H, Onizawa M, Wei L, McManus MT, Weiss A. Negative regulation of hif1a expression and th17 differentiation by the hypoxia-regulated microrna mir-210. Nat Immunol. 2014;15:393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu R, Zeng J, Yuan J, Deng X, Huang Y, Chen L, Zhang P, Feng H, Liu Z, Wang Z, Gao X, Wu H, Wang H, Su Y, Zhao M, Lu Q. Microrna-210 overexpression promotes psoriasis-like inflammation by inducing th1 and th17 cell differentiation. J Clin Invest. 2018;128:2551–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartel DP. Metazoan micrornas. Cell. 2018;173:20–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Z, Li Y, Zhang H, Huang P, Luthra R. Hypoxia-regulated microrna-210 modulates mitochondrial function and decreases iscu and cox10 expression. Oncogene. 2010;29:4362–4368 [DOI] [PubMed] [Google Scholar]
- 30.Chan SY, Loscalzo J. Microrna-210: A unique and pleiotropic hypoxamir. Cell Cycle. 2010;9:1072–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groth A, Vrugt B, Brock M, Speich R, Ulrich S, Huber LC. Inflammatory cytokines in pulmonary hypertension. Respir Res. 2014;15:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115:165–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. Rag-1-deficient mice have no mature b and t lymphocytes. Cell. 1992;68:869–877 [DOI] [PubMed] [Google Scholar]
- 34.Waypa GB, Schumacker PT. Hypoxia-induced changes in pulmonary and systemic vascular resistance: Where is the o2 sensor? Respir Physiol Neurobiol 2010;174:201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kloner RA. Clinical application of remote ischemic preconditioning. Circulation. 2009;119:776–778 [DOI] [PubMed] [Google Scholar]
- 36.Cai Z, Luo W, Zhan H, Semenza GL. Hypoxia-inducible factor 1 is required for remote ischemic preconditioning of the heart. Proc Natl Acad Sci U S A. 2013;110:17462–17467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olenchock BA, Moslehi J, Baik AH, Davidson SM, Williams J, Gibson WJ, Chakraborty AA, Pierce KA, Miller CM, Hanse EA, Kelekar A, Sullivan LB, Wagers AJ, Clish CB, Vander Heiden MG, Kaelin WG, Jr. Egln1 inhibition and rerouting of alpha-ketoglutarate suffice for remote ischemic protection. Cell. 2016;164:884–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Oka N, Tropak M, Callahan J, Lee J, Wilson G, Redington A, Caldarone CA. Remote ischemic preconditioning elaborates a transferable blood-borne effector that protects mitochondrial structure and function and preserves myocardial performance after neonatal cardioplegic arrest. J Thorac Cardiovasc Surg 2008;136:335–342 [DOI] [PubMed] [Google Scholar]
- 39.Leung CH, Wang L, Nielsen JM, Tropak MB, Fu YY, Kato H, Callahan J, Redington AN, Caldarone CA. Remote cardioprotection by transfer of coronary effluent from ischemic preconditioned rabbit heart preserves mitochondrial integrity and function via adenosine receptor activation. Cardiovasc Drugs Ther. 2014;28:7–17 [DOI] [PubMed] [Google Scholar]
- 40.Slagsvold KH, Rognmo O, Hoydal M, Wisloff U, Wahba A. Remote ischemic preconditioning preserves mitochondrial function and influences myocardial microrna expression in atrial myocardium during coronary bypass surgery. Circ Res. 2014;114:851–859 [DOI] [PubMed] [Google Scholar]
- 41.Zaccagnini G, Martelli F, Fasanaro P, Magenta A, Gaetano C, Di Carlo A, Biglioli P, Giorgio M, Martin-Padura I, Pelicci PG, Capogrossi MC. P66shca modulates tissue response to hindlimb ischemia. Circulation. 2004;109:2917–2923 [DOI] [PubMed] [Google Scholar]
- 42.Hu S, Huang M, Li Z, Jia F, Ghosh Z, Lijkwan MA, Fasanaro P, Sun N, Wang X, Martelli F, Robbins RC, Wu JC. Microrna-210 as a novel therapy for treatment of ischemic heart disease. Circulation. 2010;122:S124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eken SM, Jin H, Chernogubova E, Li Y, Simon N, Sun C, Korzunowicz G, Busch A, Bäcklund A, Österholm C. Microrna-210 enhances fibrous cap stability in advanced atherosclerotic lesions. Circ Res. 2017;120:633–644 [DOI] [PubMed] [Google Scholar]
- 44.Vasamsetti SB, Florentin J, Coppin E, Stiekema LC, Zheng KH, Nisar MU, Sembrat J, Levinthal DJ, Rojas M, Stroes ES. Sympathetic neuronal activation triggers myeloid progenitor proliferation and differentiation. Immunity. 2018;49:93–106. e107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating micrornas as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmittgen TD, Lee EJ, Jiang J, Sarkar A, Yang L, Elton TS, Chen C. Real-time pcr quantification of precursor and mature microrna. Methods. 2008;44:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghasemi A, Zahediasl S. Normality tests for statistical analysis: A guide for non-statisticians. Int J Endocrinol Metab. 2012;10:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


