FIGURE 1.
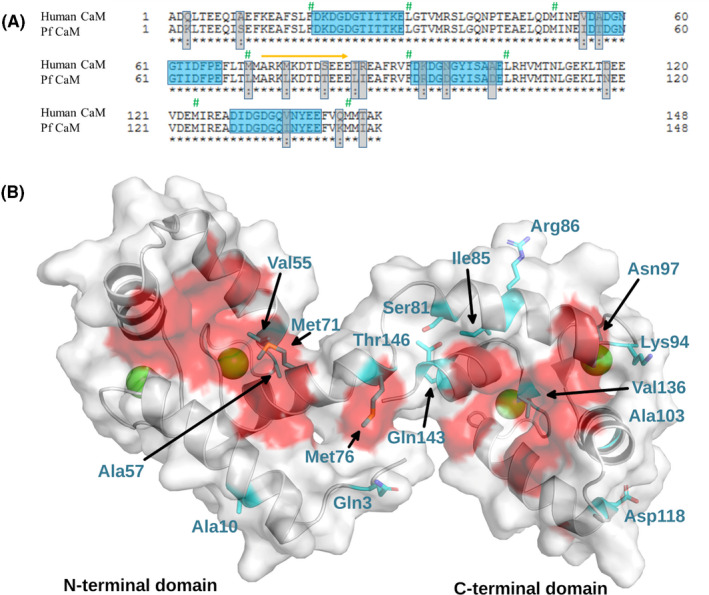
Sequence comparison of human and Pf CaM A, Sequence alignment of human and Pf CaM. Nonidentical residues and Ca2+‐binding loops are highlighted by gray and blue boxes, respectively. Similar substitutions are marked by colon. Residues forming the hydrophobic pockets are labeled by green hashes, and the flexible central linker by an orange arrow. Alignment was performed using the uniprot website (https://www.uniprot.org). B, Location of the nonidentical residues on the structure of CaM. The structure of human CaM is shown (pdb code: 2MGU). Differences between human and Pf CaM are highlighted by cyan and shown with sticks, and Ca2+ ions are shown as green balls. Hydrophobic surfaces participating in ligand binding are shown in red.
