Scheme 1. Synthetic route for compounds 1–9.
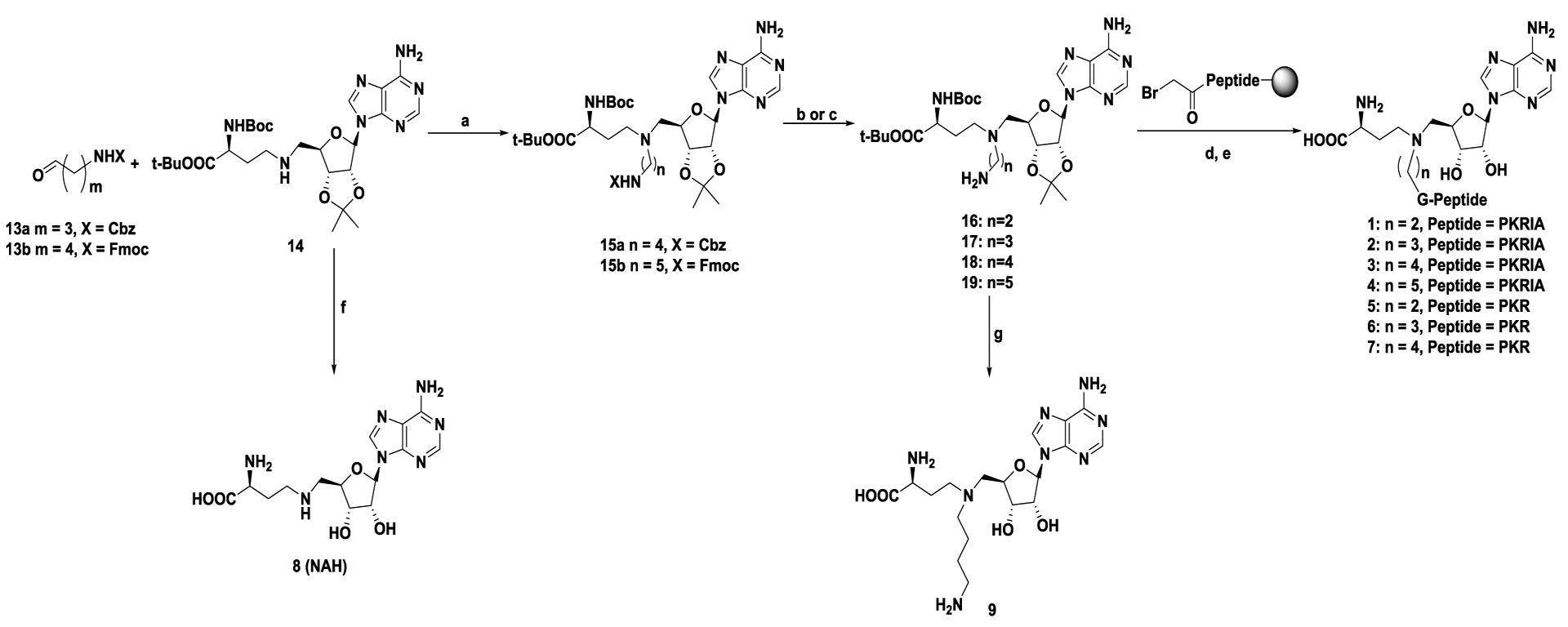
Reagents and conditions: a) NaBH3CN, CH3OH, r.t., 38–68%; b) 10% Pd/C, H2, MeOH, 56%; c) DBU, CH3CN, 0 °C to r.t., 71%; d) α-bromo peptides, DIPEA, DMF, r.t.; e) TFA: DODT: H2O: TIPS = 94: 2.5: 2.5: 1; f) TFA, CH2Cl2, 0 ℃ to r.t., 42%; g) 4N HCl in dioxane, H2O, 0 °C to r.t., 45%.
