Abstract
Background
Animal phobia is one of the most common forms of specific phobias. This anxiety disorder challenges the medical student working with animal models. Regarding this, the present study was conducted to investigate the effectiveness of one- and multi-session cognitive exposure-based treatments in students with rat phobia.
Methods
For the purpose of the study, a total of 40 female students with rat phobia were allocated into two groups of one- and multi-session cognitive exposure-based treatments. The data were collected using psychological measures, including state anxiety, rat phobia, and disgust questionnaires, which were completed in three stages, including the baseline, pre-treatment, and post-treatment. The gene expression levels of pro-inflammatory cytokines (ie, interleukin-1 [IL-1], nuclear factor-kappaB [NF-κB], and tumor necrosis factor-alpha [TNFα]) associated with acute stress, as well as the serum levels of IL-6 and cortisol, were determined using reverse transcription-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA) methods. This study was registered at the Iranian Registry of Clinical Trials (IRCT20171123037602N1).
Results
According to the results, both treatments yielded a significant reduction in almost all psychological measures and biological variables, except for IL-6. Rat phobia was the only variable that showed a statistically greater reduction in the multi-session treatment group. Furthermore, rat phobia and disgust reduction were maintained in both groups to the same extent during follow-up.
Conclusion
The findings of the present study were indicative of the incidence of habituation in psychological and biological factors following exposure therapy. Both one- and multi-session treatments reduced the factors associated with rat phobia almost to the same degree. As a result of the high levels of disgust, anxiety-related biological factors remained high in four students despite observing a significant reduction in their fear. This led to passive avoidance in this group. The OST enabled the students to handle rats in less than half a day. Accordingly, it could be applied as a half-day workshop for students in medical universities to avoid the incidence of associated anxiety-related disorders in this group.
Keywords: specific phobia, exposure therapy, gene expression, cortisol, habituation
Introduction
Specific phobia is an anxiety disorder defined as an overwhelming and irrational fear of specific objects, situations, or circumstances resulting in avoidance behavior.1 In this type of phobia, fear is much greater than the real threat. Specific phobia ranks as the most frequent form of anxiety disorder with a lifetime prevalence of 12.5%2 and a high predilection for females (ie, about twice higher than that in males).3 Animal phobia is one type of specific phobia, which results in the automatic avoidance of contact with specific animals. This avoidance can negatively affect wellbeing in some individuals by preventing them from performing or participating in education and research activities that involve animals.4 Individuals with an animal phobia might never seek treatment until their fear starts to affect their function.5,6 However, this issue should be resolved in the affected individuals, especially in the people exposed to situations where contact with an animal is inevitable, such as in those involved in the in vivo studies.
For years, animal research has played a critical role in expanding medical, veterinary, and pharmacological research. Accordingly, working with animals is an integral part of educational programs. For some students, it is inescapable to handle and work with laboratory animals during their studies. For instance, in the courses of “Practical Pharmacology” provided to the students of pharmacy and “Laboratory animals” presented to all postgraduate students, the students have to handle and work with animals. Among laboratory animals, rodents (mice and rat) are widely used in animal studies owing to a number of reasons, such as large size making them easier to handle (for sampling and performing procedures), capability to learn tasks, and possibility of inducing diseases (eg, obesity, diabetes, and cancer).7,8
Among the available treatments for specific phobia, in vivo exposure has been reported as the most potent and effective therapy, yielding the best results for the treatment of most specific phobias.9 In this method, the individual is gradually exposed to the feared stimulus on a repetitive basis, until the stimulus does not provoke fear reaction anymore. This type of exposure has been widely reported to treat specific phobias, particularly animal phobias, such as spider, snake, rat, and dog phobias.10–18
Although specific phobia can be successfully managed after a number of sessions (eight to ten sessions), several studies found that the one-session treatment (OST) developed by Lars-Göran Öst is as effective as multiple-session therapies.12,15,17,19–22 In one-session exposure-based cognitive-behavior therapy (CBT), all of the steps are performed in a long single session. On the other hand, in multiple-session exposure-based CBT, the same steps are extended and divided into several sessions.
Regardless of the treatment session numbers, the underlying mechanism of treatment is inhibitory learning, which is likely to be central to extinction.23 Based on this model, after extinction, the conditioning stimulus would possess two contrary meanings, including its original excitatory (conditioning stimulus-unconditioning stimulus) and an additional inhibitory (conditioning stimulus without unconditioning stimulus) meaning.24 Habituation is also involved as an additional mechanism in this process,25 in which the defensive response of the individual is reduced following repeated exposures to the stimulus.22,26
The experience of anxiety in an individual is followed by the incidence of physiological changes in hormone, biomarker, and gene expression levels. In this regard, when one faces a potential stressor, the central nervous system starts a series of activities that lead to the release of several neurotransmitters, peptides, and hormones.27 Two major axes involved in mediating these activities are the hypothalamic-pituitary-adrenal (HPA)28 and sympathetic nervous system.29,30 Although cortisol as the final product of HPA plays a significant role in stress responses,31 there are several other mediators, including inflammatory cytokines, involved in adaptation to stress. For instance, it has been shown that psychological stress is ultimately related to the increased synthesis of inflammatory cytokines, such as IL6,32 TNF, and IL1, in the serum or the expression of pro-inflammatory genes, such as NF-kb.33,34 On the other hand, a great body of evidence is suggestive of the critical role of excessive inflammation in the pathophysiology of stress-related diseases.
Repetitive exposure to stress is associated with adverse health consequences.35 Based on the recent epidemiological data, the experience of stressful conditions might serve as a risk factor for insulin resistance, heart disease, and obesity in women.35 The treatment process of anxiety disorders would not only reduce the amount of psychological anxiety but also lead to the incidence of habituation in biological components.36 Most of these mentioned studies have been performed in those with chronic stress or in healthy individuals during Trier Social Stress Test (TSST). Due to the scarce number of studies, the efficiency of the type of treatment in reducing the level of inflammatory biomarkers under acute stressful conditions (acute stressors) is not clearly understood.
With this background in mind, the present study was conducted to examine the effectiveness of one- and multi-session treatments with the CBT model in reducing rat phobia among students. It was hypothesized that the psychological symptoms and associated biomarkers in individuals with rat phobia would be decreased after receiving one/multi-session exposure-based treatments. This study was also targeted toward comparing the two types of treatments with respect to their effectiveness in the improvement of psychological and biological variables.
Methods
Study Population
This study was performed at the University of XXX in collaboration with XXX. By announcement in the social media groups to which the students were subscribed (eg, Telegram, WhatsApp.) and classes, volunteer female students were recruited from medical, pharmacy, and paramedical schools if meeting the eligible criteria. The inclusion criteria were: (a) age range of 18–35 years, (b) body mass index of 18–35 kg/m2, (c) self-report regarding the fear of mice or rats, and (d) diagnosis of specific phobia (mice/rat) by a registered clinical psychologist based on the DSM-5 criteria. If the interviewer had any doubts about the diagnosis of a particular subject, she consulted with her supervisor. The exclusion criteria were: (a) exposure to mice/rat or taking “Laboratory Animals” course, (b) consumption of anti-anxiety, psychoactive, beta-blocker, or hormonal and contraceptive drugs, (c) smoking habit, (d) history of psychiatric, endocrine, cardiovascular, chronic, or infectious diseases (during the last month), and (e) injection or blood phobia.
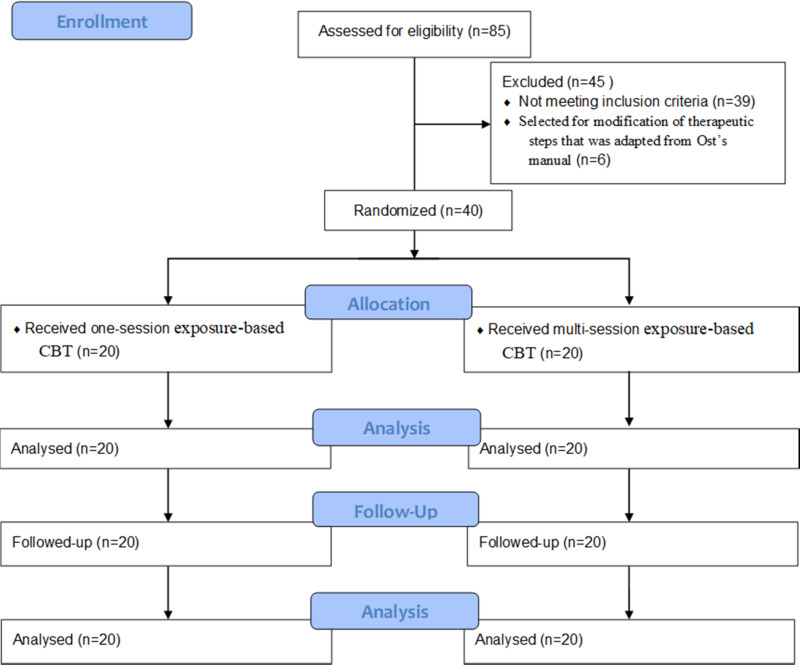
A total of 85 female students participated in the initial screening stages; however, 39 cases were excluded due to not meeting the inclusion criteria. Out of the remaining 46 cases, 6 students were randomly selected for the modification of the therapeutic steps, adapted from a manual developed by Ost. Finally, the remaining 40 students with a mean age of 20.97±1.25 years (age range: 18–24 years) met the inclusion criteria and were entered into the study. The study was approved by the Local Ethics Committees of both Shiraz University of Medical Sciences (IR.SUMS.REC.1397.245) and University of Social Welfare and Rehabilitation Sciences (IR.USWR.REC.1396.256) and performed in accordance with the 1964 Declaration of Helsinki and its later amendments. This study was also registered at the Iranian Registry of Clinical Trials (IRCT number: IRCT20171123037602N1). All participants gave written informed consent prior to their enrollment.
Psychological Measures
The instruments used in the present study had been already validated for the Persian language and population.
Diagnostic Interview
As the diagnostic criteria of specific phobia are similar in DSM-IV and DSM-5, the ADIS-IV was used to screen the students with specific phobia. The ADIS-IV is a structured interview designed to assess the current episodes of anxiety disorders.37 Moreover, this interview measures some other disorders that usually co-occur with anxiety disorders.38 The ADIS-IV has shown excellent inter-rater reliability for anxiety disorders.39 In the present study, the Persian version of ADIS-IV was adopted.40
Fear of Rat Questionnaire
This questionnaire is an adaptation of the Fear of Spiders Questionnaire (FSQ), which was modified to evaluate rat phobia. The FSQ is an 18-item self-report measure for assessing the level of spider phobia.41 For the completion of FSQ, participants are asked to rate their agreement with the questionnaire statements on a 7-point Likert scale (from 0 to 6). This tool has a score range of 0–108, with a cutoff point of ≥15 showing a midlevel of spider phobia.41 The FSQ has an adequate test-retest reliability of 0.9142 and an internal consistency of 0.97.43 This questionnaire was translated to Farsi, with the word “rat” being substituted with “spider” throughout the test. Furthermore, the format of the test was modified to make a list of questions suitable for assessing rat phobia.
In the current study, content validity ratio (CVR) and content validity index (CVI) were determined to quantitatively assess the content validity of this tool. The Lawshe’s method has been the most common technique for the evaluation of content validity. Based on the Whalts and Bassel’s method, the CVI was measured by summing up the scores of the items considered “necessary” by twenty psychologists and psychiatrists. In the next stage, the mean validity coefficient of all questions was computed to measure the total validity or the scale CVI of the questionnaire. In this study, the CVI was estimated at 0.84, which is acceptable because it is above 0.79.44
Moreover, the CVR was calculated for every single question using 20 specialists. A CVR should be more than 0.42 to be acceptable. In the present study, all questions in the questionnaire were found to be valid. To calculate the questionnaire reliability, the Fear of Rat Questionnaire (FRQ) scores of the 85 students that participated in the initial screening stage were used, rendering a Cronbach’s alpha coefficient of 0.91.
Behavioral Measure
The Behavioral Approach Test (BAT) was used to assesses to what extent the students dare to approach adult rats. The students were asked to perform these steps as shown in Table 1. In each step, the students were required to approach the rat more closely and finally pick up the rat. The participants performed the BAT at the pre-treatment and post-treatment stages. Before the test began, the therapist explained the procedure as follows:
To recognize how far you would dare to approach a rat, I will ask you to perform a number of steps. You are free to refuse any step. But you should do your very best to accomplish the step. I want you to tell me your SUDs at each step. Do you have any questions about this process?
Table 1.
Behavioral Approach Task Applied for the Treatment of Rat Phobia
| Step | Description |
|---|---|
| 1 | Approach the rat cage as close as possible. (with four adult rats in a standard small breeder animal cage with a closed door being placed on the table) |
| 2 | Touch the cage. (>10 sec) Yes/No |
| 3 | Pick up the cage. (>10 sec) Yes/No |
| 4 | Open the cage. (>10 sec) Yes/No |
| 5 | Touch the rat’s back (in the cage). Yes/No |
| 6 | Touch the rat’s tail (in the cage). Yes/No |
| 7 | Hold the rat by the palm of your hand. Yes/No |
| 8 | Pick up a rat in any way that you can. Yes/No |
After the therapist gave the instructions, each student was asked if she was willing to do the step. When a student gave up performing a step, the therapist persuaded her by asking, “Are you sure you cannot carry out this task?”.
Subjective Units of Distress Scale
The SUD was used by the students to subjectively rate the severity of their anxiety or phobia during exposure to a rat, on a scale ranging from 0 (least anxiety) to 100 (most anxiety). In this research, a range of 0–100 was considered because it covers a broader spectrum and is more precise.
Disgust Propensity and Sensitivity Scale-Revised
The Disgust Propensity and Sensitivity Scale-Revised (DPSS-R) is a 16-item tool that was developed by van Overveld et al (2006) to assess disgust occurrence (ie, disgust propensity) and the emotional impression of this experience (ie, disgust sensitivity). This scale is rated on a 5-point Likert scale, rating from 1 (never) to 5 (always). Previous research has confirmed the reliability of the DPSS-R, with the alpha coefficients of 0.78 and 0.77 for disgust propensity and disgust sensitivity subscales, respectively.45 The psychometric properties of DPSS were also assessed among the Iranian population by Zanjani et al (2016). Their results showed that this scale has an adequate factor structure, convergent validity, test-retest reliability (r=0.44), and internal consistency (α=0.83). Hence, the Persian version of DPSS-R can be used in the Iranian population. In order to investigate the convergent validity of this scale, the correlations of this scale with the Padua Inventory of obsessive-compulsive disorder symptoms and Disgust Scale-Revised were investigated, rendering the values of 0.39 and 0.51, respectively, at the significance level of 0.01.46
State-Trait Anxiety Inventory
Students’ anxiety score was assessed using the State-Trait Anxiety Inventory (STAI).47 This questionnaire contains 40 separate scales for measuring state and trait anxiety. The items of this tool are scored on a 4-point Likert scale, ranging from 1 to 4. The state anxiety scale assesses emotional response to a specific situation at the moment, while the trait anxiety scale evaluates a relatively stable disposition in response to a wide range of situations considered threatening and reflects the individual differences. In this study, the form Y of STAI was used to assess state anxiety in students.
These scales were translated into the Persian language, and their validity was verified. The validity and reliability of the Persian version of the STAI were confirmed by Mahram (1994). With regard to the internal consistency of this tool, the Cronbach’s alpha coefficients of the state and trait items were, respectively, reported as 0.91 and 0.90, and the validity of the inventory was confirmed.48 With regard to the context of Iran, Sadeghi (2002) reported the reliability coefficients of 0.93 and 0.90 for the state and trait scales of this tool, respectively.49
Biological Measures
There is evidence showing that inflammatory stress responses peak 20–30 min after exposure to stressors. Therefore, in the present study, blood samples were collected 20–30 min after student contact with the animal on both pre- and post-treatment days.50 During each sampling stage (ie, baseline, pre-treatment, and post-treatment), blood was collected into 5-mL Vacutainers, containing active gel for the isolation of serum, and 3-mL Vacutainers with EDTA as an anticoagulant for RNA isolation.
Gene Expression Analysis
Total RNA was extracted from peripheral blood samples, using the TRIzol Reagent (Invitrogen, Germany) and chloroform (Merck, Germany) according to the manufacturer’s instructions. The quantity and quality of the extracted RNA we assessed by a nanodrop spectrophotometer (Thermo Scientific, USA). The complementary DNA (cDNA) was synthesized from the DNase-treated aliquot of total RNA, utilizing the RevertAid First Strand cDNA Synthesis Kit. For quantitative real-time polymerase chain reaction (q-RT-PCR), approximately 2 μL of cDNA was amplified in each 25-μL PCR reaction mix, containing 12.5 μL of 2x SYBR Green Master Mix (Applied Biosystems, USA), 0.2 μL of each 10-pmol forward and reverse primers (designed in Primer 3 software, Table 1), and 10.1 μL DEPC water.
Gene expressions were determined using the QuantStudio real-time PCR systems (Applied Biosystems). As shown in Table 2, gene-specific primers were used for the amplification of IL-1b, TNFα, NF-kb, and TFRC and GAPDH genes (as housekeeping genes). Thermal cycling for all genes included a denaturation step at 95°C for 10 min, followed by 45 cycles that included denaturation at 95°C for 15 sec, annealing at 56°C for 30 sec, and extension at 60°C for 60 sec. The acquisition of a fluorescence signal was programmed at the end of the extension step. The accuracy of amplification was confirmed by performing melting curve analyses after each run. The samples were normalized to GAPDH and TFRC expression (as housekeeping genes), and 2−ΔΔCt values were calculated as previously described.51
Table 2.
Sequences of Primers Used for Detecting the mRNA Expressions of the Examined Genes
| 1 | NFkB_F | GCAATCATCCACCTTCATTCTCAACTT | [27] |
| 2 | NFkB_R | CCTCCACCACATCTTCCTGCTTAG | [24] |
| 3 | IL1B_F | GGCTTATTACAGTGGCAATGAGG | [23] |
| 4 | IL1B_R | GTAGTGGTGGTCGGAGATTCG | [21] |
| 5 | TFRC_F | CCTTCCTTCAATCACACTCAGTT | [23] |
| 6 | TFRC_R | ACAGTCTCCTTCCATATTCCCAAA | [24] |
| 9 | TNFα_F | AGGGCCTGTACCTCATCTACTCCCA | [25] |
| 10 | TNFα_R | AGCTGGAAGACCCCTCCCAGATAGA | [25] |
| 11 | GAPDH_F | AAGGTGAAGGTCGGAGTCAACGGA | [24] |
| 12 | GAPDH_R | TCGCTCCTGGAAGATGGTGATGGG | [24] |
Cytokine Assessment
The serum concentrations of cortisol and IL-6 were measured in the blood samples collected between 13:00 and 14:00 in duplicate using specific ELISA kits, according to the manufacturer’s instructions (QIA61, Oncogene Research Products, Cambridge, MA; DTA50 and D6050, R&D Diagnostics, Minneapolis, MN, respectively).
Treatment Conditions
The treatment sessions were conducted by a clinical psychologist and an assistant who was professionally trained in the field of working with laboratory animals. One-session treatment included a 3-hour group therapy, whereas multi-session treatment included four 2-hour group therapy sessions with weekly intervals. To design a therapeutic manual, hypothetical steps were designed by three experts (ie, principal investigator and her assistant and the supervisor) in accordance with the manual developed by Lars-Goran Ost52 to target animal phobia, with some modifications to adjust it for rat phobia.
In order to examine the designed steps, six students were selected for a three-hour therapeutic pilot session. The steps and their order were modified and rewritten based on the students’ comments and performance, observations made by the therapist and her supervisor at the session, and consultation with other specialists that watched the recorded pilot session. Finally, the designed treatment manual included 12 steps for both groups (Supplementary Table 1). In the treatment session, before each step, the students were asked to talk about their subjective units of distress (SUD) and negative and catastrophic thoughts about that step and then rate their beliefs regarding those thoughts.
In accordance with Thorpe and Salkovskis (1995), students’ cognition involved three basic aspects, namely harm (eg, “the rat will bite me”), disgust (eg, “the rat will feel fluffy”), and coping (eg, “I won’t be able to do this”).38,53 In each step, the specific information about what might be happening in reality was provided, and the therapist challenged those negative thoughts. Subsequently, the students’ SUDs and percentage of beliefs in the thoughts about the steps were recorded again. In addition, the therapist challenged these negative thoughts with behavioral tests during the task.
Before each step, the students were typically asked, “what do you think will happen if you do this?”. The therapist would then answer with “let’s see what will happen” to test the negative thought. Subsequently, the therapist assistant provided instructions and modeling to perform the step. The student then observed the assistant completing the task and then completed that task either alone or along with the therapist. The participants repeated each step until their SUD rating reached below 20. The task in each step was finalized and completed when the student was capable of doing the task by herself.
All the steps were completed in the same pattern. Each participant was allowed to ask for a break and switch her turn with another student. She resumed the exposure to fulfill the step when she was ready during her next turn. Throughout the treatment, the therapist persuaded the student to go through the steps. Although the treatment could be terminated earlier if a student expressed an extreme emotional reaction, all the treatments were completed based on the clinical judgment of the therapist. The final treatment step involved holding and handling the rats and taking a photo with the animal.
Procedure
All volunteer students who were willing to participate in this study were interviewed using the ADIS-IV to diagnose a specific phobia disorder (rat phobia) and then asked to complete the FRQ. The main interviewer was an approved clinical psychologist and asked about any previous history of psychiatric disorders in the students. For each subject, two clinical diagnoses were established by both the interviewer and her supervisor. The final participants were randomly allocated into two therapy groups, receiving either OST or multi-session treatment (Figure 1). For group assignment, each student received a number based on the order of her entrance to the study. They were then randomly assigned into two groups of 20 cases using systematic allocation. The students with odd numbers received OST, while those with even numbers were subjected to multi-session treatment. Number allocation and random assignment were performed by the secretary of the department (ie, an independent allocator).
Figure 1.
CONSORT flowchart of the study process.
First Stage: Baseline Assessments
At this stage, the baseline biomarkers and psychological symptoms (based on the State Anxiety Inventory, FRQ, and DPSS-R questionnaire) of the eligible students were measured. The completion of the State Anxiety Inventory and DPSS-R questionnaires was accomplished when the students were not involved with exams. In addition, the students’ FRQ scores, measured at the initial interview, were used for this phase (ie, baseline). Blood samples were taken from the students before encountering any rat or mouse. All laboratory measurements (in all stages) were scheduled in the afternoon (13:00–15:00) to control the circadian variation of stress hormones. The students were asked not to eat or drink anything, except water 1 h prior to the tests.
After a resting period of 30 min to ensure recovery from potential stress response to a new environment or laboratory, blood sampling was performed. Blood samples were transferred to two sterile tubes, containing EDTA anticoagulant and activating gel. Blood serum was isolated by centrifugation and then aliquoted into 0.5-mL tubes and stored at 80°C for ELISA tests. The blood samples in the anticoagulant tube were transferred to the laboratory on ice and immediately used for RNA isolation and cDNA synthesis.
Second Stage: Pre-Treatment Assessments
Approximately a week after the baseline assessment, the students were recalled for the second phase of research. At this stage, the students were separately taken to the animal lab and faced with a glass cage, containing four white adult rats (Sprague Dawley weighing 200–250 g) placed on a table for about 5–7 min in the presence of laboratory animal technician and therapist assistant as an evaluator. The participants had to face the rats in accordance with the eight BAT steps. The students’ ability to take each step was recorded (yes/no), and the degree of fear in that step was measured on the basis of SUDs.
Since it was important for us to measure the true level of anxiety when facing the rats, the students were asked to approach as much as they could (even with anxiety); however, they were not obligated to complete the tasks. Whenever the SUDs of the student reached 100, the exposure stopped, and they were guided to the next room for blood sampling and psychological assessments (ie, STAI, FRQ, and DPSS-R).
Third Stage: Therapeutic Intervention
At this stage, according to the previous categorization, the students were placed into two groups of OST and multi-session treatment. For both types of treatments, therapy was performed in four groups each of which contained five students. The OST and the first session of the multi-session treatment were performed after the initial exposure (ie, second step) so that the students would not return home with anxiety. To reduce the anxiety of initial exposure and due to the lengthiness of the treatment sessions, lunch was provided before the initiation of the treatment session.
Fourth Stage: Post-Treatment Assessments
At this stage, the students were confronted with animals a week after the last treatment session. Similar to the second stage (ie, initial exposure), BAT, blood sampling and psychological evaluation (DPSS-R, STAI, and FSQ) were carried out in the same manner.
Fifth Stage: Follow-Up
Six months after the completion of the treatment, the levels of rat phobia and disgust were measured in all students in both groups by a questionnaire. Since blood samples were taken from each student in triplicates during the study, the follow-up stage was solely conducted by FRQ and DPSS-R.
Statistical Analysis
Student age and all other variables at the baseline were compared between the groups using the independent sample t-test. In addition, repeated measures ANOVA was used to determine the effect of one- and multi-session treatments on psychological and biological variables with Sidak posthoc test. The data were expressed as mean±SD. To determine whether biological and psychological variables were habituated after the treatment, changes in the variables between the pre- and post-treatment stages were calculated and compared by independent sample t-test. In case the delta (pre-treatment value minus post-treatment value) of a variable was not normally distributed, the Mann–Whitney U-test was used instead of the independent sample t-test. Moreover, if the baseline measure of a variable was statistically significant between the two groups, ANCOVA was used to compare that variable between the two treatment methods for controlling the baseline score. All statistical analyses were performed in SPSS software, version 22 (IBM, USA). A p-value less than 0.05 was considered statistically significant.
Results
In this study, the mean ages of the female students in the one- and multi-session treatment groups were obtained as 21.15±1.38 and 20.8±1.1 years, respectively. Accordingly, the two groups were comparable in terms of age. Moreover, the baseline measures were not significantly different between the two groups, except for rat phobia. In this regard, the mean scores of rat phobia were obtained as 86.05±20.91 and 99.25±15.14 in one-session and multi-session treatment groups, respectively (t(38)=−2.28, P<0.05). Although the mean score of disgust was higher in the multi-session group (45.15±7.17) than in the one-session group (49.45±6.41), this difference was not statistically significant (P=0.053). In the second stage (ie, pre-treatment) of the research, the students were only able to perform the first few steps of BAT, while in the fourth stage (ie, post-treatment), they performed the steps with success and low anxiety. In this regard, all 40 students successfully completed the treatment and took a picture at the end while handling a rat.
Effect of One- and Multi-Session Treatments on Psychological and Biological Variables
The examined psychological and biological variables in the one- and multi-session groups were evaluated in three stages, including baseline, pre-treatment, and post-treatment. Moreover, rat phobia and disgust were measured 6 months after the termination of the treatment for the fourth time as a follow-up stage. The repeated measures ANOVA was used to assess the efficacy of both treatments in the reduction of the variables (Table 3). Because of the interaction, repeated measure was used for the assessment of rat phobia and NF-kB (Table 4). Based on the results of the posthoc analysis, all variables, except for the self-expressions associated with disgust and rat phobia, showed a significant increase in both groups after facing rats in comparison to the baseline data. Furthermore, the comparison of pre- and post-treatment results indicated that except for IL-6, all other variables had a significant decrease in the two groups, and habituation occurred both biologically and psychologically. However, during the follow-up phase, the level of rat phobia significantly increased in both treatment groups, compared to that in the post-treatment stage (Table 4). Yet, rat phobia level was statistically lower than those estimated at the baseline and pre-treatment stage. However, the level of disgust remained constant over both post-treatment and follow-up stages.
Table 3.
Effect of One- and Multi-Session Treatments on Psychological and Biological Variables in Female Students
| Variables | Time | Groups |
F (df1, df2) P-value |
Post hoc (P) | ||||
|---|---|---|---|---|---|---|---|---|
| One-Session | Multi-Session | Effect of Group | Effect of Time | Effect of Interaction | ||||
| Cortisol | a | 205.07±58.52 | 219.18±72.32 | 0.66 (1, 38) P=0.42 |
28.67 (1.53, 58.28) P=0.0001 |
0.35 (1.53, 58.28) P=0.64 |
a,b** (0.0001) a,c* (0.001) b,c** (0.0001) |
|
| b | 314.48±125.16 | 352.62±146.9 | ||||||
| c | 267.55±83.93 | 282.8±116.4 | ||||||
| Interleukin-6 | a | 142.55±122.24 | 194.11±198.21 | 0.97 (1, 38) P=0.33 |
14.73 (1.8, 68.53) P=0.0001 |
0.23 (1.8, 68.53) P=0.76 |
a,b** (0.0001) a,c* (0.001) b,c (0.99) |
|
| b | 155.29±123.44 | 210.30±204.92 | ||||||
| c | 156.58±129.93 | 207.86±206.80 | ||||||
| State anxiety | a | 43.1±9.1 | 41.6±9.06 | 0.002 (1, 38) P=0.96 |
96.72 (1.94, 74.07) P=0.0001 |
1.67 (1.94, 74.07) P=0.19 |
a,b** (0.0001) a,c** (0.0001) b,c** (0.0001) |
|
| b | 53.95±10.89 | 57.8±7.23 | ||||||
| c | 31.05±9.43 | 28.5±4.91 | ||||||
| Disgust | a | 45.15±7.17 | 49.45±6.41 | 5.86 (1, 38) P=0.02 |
31.12 (2.3, 87.45) P=0.0001 |
0.26 (2.3, 87.45) P=0.79 |
a,b (0.76) a,c** (0.0001) a,d** (0.0001) b,c** (0.0001) b,d** (0.0001) c,d (0.94) |
|
| b | 45.4±8.58 | 51.25±7.46 | ||||||
| c | 36.35±8.49 | 41.1±8.8 | ||||||
| d | 37.95±7.76 | 41.6±7.27 | ||||||
| Rat phobia | a | 68.05±20.91 | 81.25±15.14 | 1.6 (1, 38) P=0.21 |
231.81 (2.44, 92.81) P=0.0001 |
5.77 (2.44, 92.81) P=0.002 |
N/A | |
| b | 71.6±23.74 | 86.65±12.41 | ||||||
| c | 18.65±20.44 | 17.6±12.11 | ||||||
| d | 32.95±22.56 | 29±14.36 | ||||||
| Interleukin-1 | a | 1±0 | 1±0 | 2.51 (1, 38) P=0.12 |
28.56 (1.27, 48.31) P=0.0001 |
1.81 (1.27, 48.31) P=0.18 |
a,b** (0.0001) a,c (0.09) b,c** (0.0001) |
|
| b | 3.9±2.65 | 2.68±2.54 | ||||||
| c | 1.63±0.97 | 1.16±1.27 | ||||||
| TNFα | a | 1±0 | 1±0 | 1.77 (1, 38) P=0.19 |
13.85 (1.15, 43.91) P=0.001 |
0.83 (1.15, 43.91) P=0.38 |
a,b* (0.001) a,c (0.06) b,c* (0.003) |
|
| b | 4.05±4.07 | 2.80±3.73 | ||||||
| c | 1.86±1.4 | 1.12±1.17 | ||||||
| NF-kB | a | 1±0 | 1±0 | 18.19 (1, 38) P=0.0001 |
31.1 (1.91, 72.85) P=0.0001 |
10.96 (1.91, 72.85) P=0.0001 |
N/A | |
| b | 3.48±1.71 | 1.7±0.97 | ||||||
| c | 2.48±1.65 | 1.04±0.66 | ||||||
Notes: aBaseline; bPre-treatment; cPost-treatment; dFollow up; *P<0.01, **P<0.001, N/A: not applicable.
Table 4.
Effect of One- and Multi-Session Treatment on Rat Phobia Scores and Gene Expression of NF-kB
| Variables | Groups | Stage | Statistics | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline (a) | Pre-treatment (b) | Post-treatment (c) | Follow-up (d) | F (df1, df2) |
P | Post Hoc (p) | ||
| Rat phobia | One-session | 68.05 ± 20.91 | 71.6 ± 23.74 | 18.65 ± 20.44 | 32.95 ± 22.56 | 72.140 (2.05, 39.04) |
0.0001 | a,b (0.4) a,c** (0.0001) ad** (0.0001) b,c ** (0.0001) b,d** (0.0001) c,d** (0.0001) |
| Multi-session | 81.25 ± 15.14 | 86.65 ± 12.41 | 17.6 ± 12.11 | 29 ± 14.36 | 183.39 (2.26, 43.07) |
0.0001 | a,b (0.14) a,c** (0.0001) a,d** (0.0001) b,c** (0.0001) b,d** (0.0001) c,d* (0.001) |
|
| NF-KB | One-session | 1 ± 0 | 3.48 ± 1.71 | 2.48 ± 1.65 | ND | 24.57 (1.89, 36.01) |
0.0001 | a,b** (0.0001) a,c* (0.001) b,c* (0.005) |
| Multi-session | 1 ± 0 | 1.7 ± 0.97 | 1.04 ± 0.66 | ND | 8.54 (1.7, 32.32) |
0.002 | a,b* (0.005) a,c (0.75) b,c* (0.003) |
|
Notes: a: Baseline; b: Pre-treatment; c: Post-treatment; d: Follow up; *p<0.01, **p<0.001.
Despite the achievement of success in both treatment groups in handling the rats, no remarkable reduction was found in the biological variables (ie, cortisol, IL-1, Nfкb, IL-6, TNF-α) at the post-treatment stage, compared to those estimated at the pre-treatment phase in neither the three students receiving OST nor the two students participating in multi-session therapy. With respect to the psychological factors, rat phobia and state anxiety were notably reduced in these five students, while disgust was slightly mitigated in one of them.
Comparison of the Effectiveness of Two Therapeutic Methods
To compare the efficacy of the mentioned therapeutic methods, changes occurring in the variables after the treatment were calculated. Then, the variation rate of each variable was compared between the single- and multi-session treatment groups by independent sample t-test (Table 5). Among the studied variables, only the deltas (ie, pre-treatment value minus post-treatment value) of TNFα and IL-1 were not normally distributed. As a result, the Mann–Whitney U-test was used instead of the independent sample t-test.
Table 5.
Mean and Variation Rate of Variables in Students with Rat Phobia Based on the Type of Treatment
| Variables | Rate of Variation (Changes from Pre-Treatment to Post-Treatment) |
T (Changes) |
P (df=38) |
|
|---|---|---|---|---|
| One-Session (Mean±SD) n=20 | Multi-Session (Mean±SD) n=20 | |||
| Cortisol | 46.93±78.38 | 69.82±103.1 | −0.790 | 0.43 |
| Interleukin-6 | −1.29±20.31 | 2.44±19.54 | −0.59 | 0.55 |
| State anxiety | 22.9±12.94 | 29.3±8.84 | −1.82 | 0.076 |
| Disgust | 9.05±7.52 | 10.15±9.05 | −0.41 | 0.679 |
| Interleukin-1 | 2.26±2.49 | 1.52±1.7 | 1.1 | 0.27 |
| TNFα | 2.18±3.74 | 1.68±3.01 | 0.47 | 0.63 |
| NF-kB | 0.99±1.39 | 0.65±0.87 | 0.92 | 0.35 |
The mean levels of IL-1 in one- and multi-session treatment groups were 22.5 and 18.5, respectively. With regard to TNFα, these mean values were obtained as 21.6 and 19.4, respectively. The distributions of IL-1 (Mann–Whitney U=160, n1=n2=20, P=0.27 two-tailed) and TNFα (Mann–Whitney U=178, n1=n2=20, P=0.55 two-tailed) were not significantly different between the two groups. As mentioned previously, the baseline measures were not statistically different between the two groups for any variables, except for rat phobia. Therefore, ANCOVA was used to compare rat phobia between the two treatment methods for controlling the baseline score of this variable (Table 6).
Table 6.
Analysis of Covariance Showing the Difference in Rat Phobia Scores from Pre-Treatment to Post-Treatment (Change), N1=N2=20
| Variables | B | Std. Error | F | P | |
|---|---|---|---|---|---|
| Rat Phobia change | Rat Phobia Baseline score | 0.15 | 0.17 | 0.79 | 0.37 |
| Type of treatment | −14.01 | 6.7 | 4.37 | 0.04 | |
Although the reduction in the levels of most of the variables was greater in the multi-session treatment group, only the reduction of the fear of rat was statistically significant in the multi-session treatment group, compared to that in the OST group. The levels of rat phobia and disgust were compared between the baseline and follow-up, pre-treatment and follow-up, and post-treatment and follow-up stages, in the two treatment groups. Among these, only the reduction of rat phobia between baseline and follow-up (t(38)=−2.69, P=0.01) and between pre-treatment and follow-up (t(38)=−3.1, P=0.004) was significantly greater in the multi-session treatment group, compared with that in the OST group.
Discussion and Conclusion
Several review studies showed that in vivo exposures are more effective in the treatment of animal phobia than other modes of exposure.9 Unlike other disorders, a specific phobia can be treated by in vivo exposure-based methods even in one session.54 In the current study, the results revealed the considerable effect of OST on reducing rat phobia, which is in agreement with other previous reports.20,55 However, in vivo therapies have a number of shortcomings, including high dropout and acceptance rates.9 Nonetheless, there was no dropout in our study since the students required to be treated because of their need for working with rats in their practical classes.
Effect of One- and Multi-Session Treatments on Psychological Variables
In the present study, all subjects were able to handle the rats, regardless of the type of treatment. Accordingly, both treatments had a significant effect on alleviating the symptoms of rat phobia. This improvement was recorded by taking a digital photo of the participants while handling a rat. To the best of our knowledge, there is limited information regarding rat phobia. In a case study performed by Lars-Goran Ost (1987), a 24-year-old woman who had cat, snake, rat, and worm phobias was reported to be successfully treated with OST.19 In another case study, an 18-year-old female student with rat phobia was treated by four-session CBT.56 Similar to our study, the two mentioned cases were successfully treated by one- and multi-session therapies, respectively.
In the present study, to challenge students’ negative and catastrophic thoughts about each step, their fears were identified. In line with a study performed by Hisa (2003), inability to control rats and their sudden movement were raised as the most frequent causes of fear by the students. Similarly, in a case report by Ost (1987), the main causes of unpleasant feelings and fear were very rapid moving, running everywhere, and having no control over the rat. Moreover, some of our participants believed that the rat might harm or bite them, which is in line with the results reported by Hsia (2003). Additionally, rat screaming, when picked up by the scruff of the neck, was regarded as a terrifying experience by our students.
In the current study, all students in both groups successfully handled the rat and took a picture with it after the implementation of the treatment. However, biological factors associated with phobia remained relatively high in five students (ie, three students in the OST group and two cases in the multi-session group) despite a significant reduction in their fear of rat. However, the level of disgust still remained high in four of these students (ie, two students in each group). According to the literature, disgust has an important role in some specific phobias, including animal phobia.57 The students in our study expressed different levels of disgust about rats. The major causes that students reported for being disgusted with rats were the sense of their soft, wet, and slimy tail and loose skin, as well as feeling their heartbeat, breathing, and shivering while handling them.
Fear and disgust are two different emotions58 that are equally elevated in an individual who has animal phobia.45,59 According to Olatunji and Deacon (2008), fear and disgust play two different and independent roles in animal phobia. It seems that disgust is mostly related to avoidance, while fear is mostly associated with general distress about the stimulus.60 Fear causes a rapid physiological response (eg, an increase in pulse rate), which leads to quick avoidance of the stimulus. On the other hand, disgust leads to more controlled reactions, such as facial expression and cognitive processes, which result in the passive avoidance of the stimulus.61
Three possible mechanisms have been proposed for the role of disgust in some anxiety disorders, such as animal phobia. First, disgust might cause anxiety responses through information processing bias. A person has a negative and threatening interpretation of the stimuli when he/she feels disgusted.62,63 The second mechanism is an attentional bias toward the disgust stimuli. Those having this bias might experience excessive difficulty in detaching their attention away from the disgust stimuli in comparison to fear stimuli.64,65 Finally, as the Matchett and Davey’s model of small animal phobias (1991) proposed, the dread of contagion is another mechanism through which disgust might cause anxiety.66 As mentioned previously, disgust can cause anxiety in pathways that are independent of those of fear. In this study, consistent with the aforementioned research, in four students with a high level of disgust, the level of anxiety-related biological factors remained high, despite a significant reduction in their fear. On the other hand, the inhibitory learning model might provide another explanation why some biological variables remained sensible to the presence of a phobic stimulus although the participants increased their ability to cope with rats. Based on this model, even after extinction, a part of original conditioning still remains.24
Effect of One- and Multi-Session Treatments on Biological Variables
There are complementary data suggesting that the plasma levels of inflammatory molecules, such as IL-6, TNFα, NF-κB, and IL-1β, are elevated as a result of stress.67,68 Consequently, these molecules might play a role in converting stress into the risk of diseases associated with inflammation.67,68 As predicted, TNFα, NF-κB, and IL-1β gene expression in both groups increased significantly in response to an initial stressor and post-treatment habituation. These findings are in line with the results of some studies revealing a considerable stress-related increase in the expression of inflammatory genes in response to acute stress and the habituation of responses after repeated exposures.36,50
Furthermore, our findings are consistent with those of previous studies indicating the elevation of immune-related responses following acute stress and an increase in the serum concentrations of cortisol and IL-6 when encountering initial stressors.36,69–73 Similar to several studies,36,74,75 in the current research, the serum concentration of cortisol was reduced at the end of both types of exposure-based CBTs. However, the concentration of IL-6 remained elevated after repeated exposures, indicating the sensitization of IL-6 following repeated exposures to acute stressors, which is in accordance with previous studies.36,70,73 The NF-κB is a well-known regulator of the immune response and a target for the adjustment of stress-related signals.76 This molecule is found in cells in an inactive form and can be rapidly induced by cellular stimuli, such as TNF-α and IL-1β.77,78 In this context, the results of the present research revealed a correlation between the expression of IL-1b (r=0.55, P< 0.0001) and TNF-α (r=0.39, P<0.0001) with NF-κB, which is mostly comparable to the results obtained by Brydon et al (2005).
Comparison of the Effect of Two Therapeutic Methods for Reducing Rat Fear
The literature contains limited studies comparing the outcome of OST and standard multi-session CBT-based treatment for specific phobia. In this regard, studies on adults have only targeted blood and injection phobia,54,79 flying phobia,55 and claustrophobia.80 In the current research, the only variable in which multi-session treatment showed superiority over OST was rat phobia since it induced a better clinical improvement in this regard. However, the two methods were almost comparable in terms of the changes they induced in other variables. These findings are in agreement with those of a study that indicated the advantages of multi-session treatment over OST for the management of blood phobia.54
In the present study, the comparison of the changes in state anxiety showed a greater numerical reduction during the multi-session treatment; however, it was not statistically significant. However, the comparison of one- and multi-session treatments for the management of injection phobia79 and claustrophobia80 revealed no significant difference between the two therapeutic methods. The OST is more cost-effective and takes less time, compared to multi-session treatment. However, one of the disadvantages of OST observed in the current research was that the students were under a relatively high pressure to take the therapeutic steps because of time limitation. One of the things that reduced this pressure was the implementation of group therapy. This measure increased students’ motivation as a result of observing the success of others and gaining better observational learning.
Among the studied variables, only rat phobia and disgust were assessed during the follow-up stage. The results revealed no significant difference in the level of disgust between the post-treatment and follow-up stages in both groups. In other words, the effectiveness of the two methods in reducing disgust remained stable after 6 months. In the same vein, there was no significant difference between post-treatment and follow-up stages regarding the level of rat phobia in both types of treatments. These results are comparable to those of previous studies indicating the sustainability of response to the exposure-based treatment of animal phobia for at least 6 months.12,15–17,81,82 Similarly,54 the response to treatment was preserved in both treatment groups to the same degree at the follow-up stage. However, this finding was not in accordance with the other previously published data indicating the advantage of multi-session treatment over OST at this stage.55,83
A limited number of studies have measured the reduction of inflammatory biomarkers as the indicators of the effectiveness of repeated exposures to a phobic situation. Almost all of these studies were performed on normal individuals without using any therapeutic interventions. The results of this study showed that habituation following exposure-based treatment can occur not only in behavioral and psychological characteristics but also in the serum cortisol and cellular levels of inflammatory biomarkers. Although the reduction of rat phobia was greater in the multi-session therapy group than in the OST group, the final result, which was handling the rats, was similar in both types of treatments. Since in OST, the students were able to handle the animal in less than half a day, it could be applied as a half-day workshop in medical universities for applicants. This will reduce anxiety-related physical and psychological disorders in the students who have to handle rats in their practical courses. Moreover, it enables pharmacy and postgraduate students to work on animal models. Another new finding of the present study was the possible role of disgust in maintaining biomarkers at a high level, which was observed in a number of students.
Given the time- and cost-effectiveness of OST, investigating the usefulness of this method in reducing the fear of pets, such as cats and dogs, along with assessing additional biological and psychological components, is warranted. It should be noted that there is a non-inferiority randomized controlled trial comparing the Ost’s OST with multi-session CBT treatment among 286 children aged 7–16 years with all types of specific phobias (Wright et al, 2018).
Acknowledgments
The authors would like to express their deepest appreciation to Professor Ost for his generous provision of the treatment manual. The assistance of Sara Khanjari, Zahra Zare, Mina Bakhtiari, and Hasti Nouraei in collecting blood samples should be also appreciated. We also extend our gratitude to Maryam Shojaei and Sara Khanjari for their contribution to performing all the biological experiments and Kamran Gorgi for helping us with the pilot treatment. We greatly acknowledge Dr. Peyman Jafari for his statistical consultation. In addition, the authors wish to thank Mr. H. Argasi at the Research Consultation Center of Shiraz University of Medical Sciences for his invaluable assistance in editing this manuscript. This work was financially supported by the University of Social Welfare and Rehabilitation Sciences (Grant No. 2037) .
Data Sharing Statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymized data may be granted following review.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.APA. Diagnostic and Statistical Manual of Mental Disorders. 4 ed. Arlington, VA, US: American Psychiatric Publishing, Inc; 1994. [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- 3.Craske M, Barlow D, Clark D, et al. Specific (simple) phobia. DSM-IV Sourcebook. 1996;2:473–506. [Google Scholar]
- 4.Magee W, Eaton W, Wittchen H. Agoraphobia, simple phobia, and social phobia in the national comorbidity survey. Year Bk Psychiatr Appl Ment Health. 1997;1997:369. [DOI] [PubMed] [Google Scholar]
- 5.Stinson FS, Dawson DA, Chou SP, et al. The epidemiology of DSM-IV specific phobia in the USA: results from the national epidemiologic survey on alcohol and related conditions. Psychol Med. 2007;37(7):1047–1059. doi: 10.1017/S0033291707000086 [DOI] [PubMed] [Google Scholar]
- 6.Fink G. Stress: Concepts, Cognition, Emotion, and Behavior: Handbook of Stress Series. Vol. 1 Academic Press; 2016. [Google Scholar]
- 7.Badyal DK, Desai C. Animal use in pharmacology education and research: the changing scenario. Indian J Pharmacol. 2014;46(3):257. doi: 10.4103/0253-7613.132153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Britain G, Commission CQ. The State of Health Care and Adult Social Care in England in 2011/12. Stationery Office; 2012. [Google Scholar]
- 9.Choy Y, Fyer AJ, Lipsitz JD. Treatment of specific phobia in adults. Clin Psychol Rev. 2007;27(3):266–286. doi: 10.1016/j.cpr.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 10.Foa EB, Blau JS, Prout M, Latimer P. Is horror a necessary component of flooding (implosion)? Behav Res Ther. 1977;15(5):397–402. doi: 10.1016/0005-7967(77)90043-2 [DOI] [PubMed] [Google Scholar]
- 11.Gauthier J, Marshall W. The determination of optimal exposure to phobic stimuli in flooding therapy. Behav Res Ther. 1977;15(5):403–410. doi: 10.1016/0005-7967(77)90044-4 [DOI] [PubMed] [Google Scholar]
- 12.Hellström K, Öst L-G. One-session therapist directed exposure vs two forms of manual directed self-exposure in the treatment of spider phobia. Behav Res Ther. 1995;33(8):959–965. doi: 10.1016/0005-7967(95)00028-V [DOI] [PubMed] [Google Scholar]
- 13.Hepner A, Cauthen NR. Effect of subject control and graduated exposure on snake phobias. J Consult Clin Psychol. 1975;43(3):297. doi: 10.1037/h0076740 [DOI] [PubMed] [Google Scholar]
- 14.Muris P, Mayer B, Merckelbach H. Trait anxiety as a predictor of behaviour therapy outcome in spider phobia. Behav Cogn Psychother. 1998;26(1):87–91. doi: 10.1017/S1352465898000095 [DOI] [Google Scholar]
- 15.Öst L-G. One-session group treatment of spider phobia. Behav Res Ther. 1996;34(9):707–715. doi: 10.1016/0005-7967(96)00022-8 [DOI] [PubMed] [Google Scholar]
- 16.L-G Ö, Ferebee I, Furmark T. One-session group therapy of spider phobia: direct versus indirect treatments. Behav Res Ther. 1997;35(8):721–732. doi: 10.1016/S0005-7967(97)00028-4 [DOI] [PubMed] [Google Scholar]
- 17.L-G Ö, Salkovskis PM, Hellström K. One-session therapist-directed exposure vs. self-exposure in the treatment of spider phobia. Behav Ther. 1991;22(3):407–422. doi: 10.1016/S0005-7894(05)80374-0 [DOI] [PubMed] [Google Scholar]
- 18.Rentz TO, Powers MB, Smits JA, Cougle JR, Telch MJ. Active-imaginal exposure: examination of a new behavioral treatment for cynophobia (dog phobia). Behav Res Ther. 2003;41(11):1337–1353. doi: 10.1016/S0005-7967(03)00041-X [DOI] [PubMed] [Google Scholar]
- 19.Öst L-G. One-session treatments for a case of multiple simple phobias. Cogn Behav Ther. 1987;16(4):175–184. [Google Scholar]
- 20.Öst L-G. One-session treatment for specific phobias. Behav Res Ther. 1989;27(1):1–7. doi: 10.1016/0005-7967(89)90113-7 [DOI] [PubMed] [Google Scholar]
- 21.Öst L-G. Rapid treatment of specific phobias In: Davey GCL, editor. Phobias: A handbook of Theory, Research, and Treatment. New York: John Wiley and Sons; 1997. [Google Scholar]
- 22.Zlomke K, Davis TE 3rd. One-session treatment of specific phobias: a detailed description and review of treatment efficacy. Behav Ther. 2008;39(3):207–223. doi: 10.1016/j.beth.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 23.Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114(1):80. doi: 10.1037/0033-2909.114.1.80 [DOI] [PubMed] [Google Scholar]
- 24.Craske MG, Treanor M, Conway CC, Zbozinek T, Vervliet B. Maximizing exposure therapy: an inhibitory learning approach. Behav Res Ther. 2014;58:10–23. doi: 10.1016/j.brat.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12(2):120–150. [DOI] [PubMed] [Google Scholar]
- 26.Marks I, Tobena A. Learning and unlearning fear: a clinical and evolutionary perspective. Neurosci Biobehav Rev. 1990;14(4):365–384. doi: 10.1016/S0149-7634(05)80059-4 [DOI] [PubMed] [Google Scholar]
- 27.Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10(6):459–466. doi: 10.1038/nrn2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Besharat MA, Behpajooh A, Poursharifi H, Zarani F. Personality and chronic fatigue syndrome: the role of the five-factor model. Asian J Psychiatr. 2011;4(1):55–59. doi: 10.1016/j.ajp.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 29.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve – an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52(4):595–638. [PubMed] [Google Scholar]
- 30.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389 [DOI] [PubMed] [Google Scholar]
- 31.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355. doi: 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- 32.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci. 2003;100(15):9090–9095. doi: 10.1073/pnas.1531903100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8(9):R189. doi: 10.1186/gb-2007-8-9-r189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller GE, Chen E, Sze J, et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-κB signaling. Biol Psychiatry. 2008;64(4):266–272. doi: 10.1016/j.biopsych.2008.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy MO, Loria AS. Sex-specific effects of stress on metabolic and cardiovascular disease: are women at higher risk? Am J Physiol Regul Integr Comp Physiol. 2017;313(1):R1–r9. doi: 10.1152/ajpregu.00185.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McInnis CM, Wang D, Gianferante D, et al. Response and habituation of pro- and anti-inflammatory gene expression to repeated acute stress. Brain Behav Immun. 2015;46:237–248. doi: 10.1016/j.bbi.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown TA, Barlow DH, DiNardo PA. Anxiety Disorders Interview Schedule for DSM-IV (ADIS-IV): Client Interview Schedule. Graywind Publications Incorporated; 1994. [Google Scholar]
- 38.Koch EI, Spates CR, Himle JA. Comparison of behavioral and cognitive-behavioral one-session exposure treatments for small animal phobias. Behav Res Ther. 2004;42(12):1483–1504. doi: 10.1016/j.brat.2003.10.005 [DOI] [PubMed] [Google Scholar]
- 39.Brown TA, Di Nardo PA, Lehman CL, Campbell LA. Reliability of DSM-IV anxiety and mood disorders: implications for the classification of emotional disorders. J Abnorm Psychol. 2001;110(1):49. doi: 10.1037/0021-843X.110.1.49 [DOI] [PubMed] [Google Scholar]
- 40.Mohammadi A, Birashk B, Gharaie B. Comparison of the effect of group transdiagnostic therapy and group cognitive therapy on anxiety and depressive symptoms. Iran J Public Health. 2013;42(1):48. [PMC free article] [PubMed] [Google Scholar]
- 41.Cochrane A, Barnes-Holmes D, Barnes-Holmes Y. The perceived-threat behavioral approach test (PT-BAT): measuring avoidance in high-, mid-, and low-spider-fearful participants. Psychol Rec. 2008;58(4):585–596. doi: 10.1007/BF03395639 [DOI] [Google Scholar]
- 42.Muris P, Merckelbach H. A comparison of two spider fear questionnaires. J Behav Ther Exp Psychiatry. 1996;27(3):241–244. doi: 10.1016/S0005-7916(96)00022-5 [DOI] [PubMed] [Google Scholar]
- 43.Wagener AL, Zettle RD. Targeting fear of spiders with control-, acceptance-, and information-based approaches. Psychol Rec. 2011;61(1):77–91. doi: 10.1007/BF03395747 [DOI] [Google Scholar]
- 44.Ayre C, Scally AJ. Critical values for Lawshe’s content validity ratio: revisiting the original methods of calculation. Meas Eval Couns Dev. 2014;47(1):79–86. doi: 10.1177/0748175613513808 [DOI] [Google Scholar]
- 45.van Overveld M, de Jong PJ, Peters ML. Differential UCS expectancy bias in spider fearful individuals: evidence toward an association between spiders and disgust-relevant outcomes. J Behav Ther Exp Psychiatry. 2006;37(1):60–72. doi: 10.1016/j.jbtep.2005.09.007 [DOI] [PubMed] [Google Scholar]
- 46.Zanjani Z, Yaghubi H, Fata L, Shairi M, Gholami Fesharaki M. Psychometric properties of disgust propensity and sensitivity scale in Iranian sample. Clin Psychol Pers. 2016. [Google Scholar]
- 47.Spielberger C, Gorsuch R, Lushene R, Vagg P, Jacobs G. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 48.Mahram B. Spielberger test Standardization in Mashhad[master thesis]. Tehran, Iran: Allameh Tabatabaei University; 1994. [Google Scholar]
- 49.Sadeghi S. Standardization Spielberger Test [master thesis]. Tehran, Iran: Islamic Azad University; 2002. [Google Scholar]
- 50.Marsland AL, Walsh C, Lockwood K, John-Henderson NA. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain Behav Immun. 2017;64:208–219. doi: 10.1016/j.bbi.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 52.Öst L-G. Manual for the 1-Session Treatment of Specific Phobias 2010. Located at: Unpublished Manuscript. Stockholm, Sweden: Department of Psychology, Stockholm University; 1997. [Google Scholar]
- 53.Thorpe SJ, Salkovskis PM. Phobic beliefs: do cognitive factors play a role in specific phobias? Behav Res Ther. 1995;33(7):805–816. doi: 10.1016/0005-7967(95)00022-P [DOI] [PubMed] [Google Scholar]
- 54.Hellström K, Fellenius J. One versus five sessions of applied tension in the treatment of blood phobia. Behav Res Ther. 1996;34(2):101–112. doi: 10.1016/0005-7967(95)00060-7 [DOI] [PubMed] [Google Scholar]
- 55.L-G Ö, Brandberg M, Alm T. One versus five sessions of exposure in the treatment of flying phobia. Behav Res Ther. 1997;35(11):987–996. doi: 10.1016/S0005-7967(97)00077-6 [DOI] [PubMed] [Google Scholar]
- 56.Hisa CC. Four day cognitive behavioral treatment of a rat phobic. Cuad Hispanoam Psicol. 2003;3(1):17–22. [Google Scholar]
- 57.Olatunji BO, Cisler J, McKay D, Phillips ML. Is disgust associated with psychopathology? Emerging research in the anxiety disorders. Psychiatry Res. 2010;175(1–2):1–10. doi: 10.1016/j.psychres.2009.04.007 [DOI] [PubMed] [Google Scholar]
- 58.Smits J, Telch M, Randall P. An examination of the decline in fear and disgust during exposure-based treatment. Behav Res Ther. 2002;40(11):1243–1253. doi: 10.1016/S0005-7967(01)00094-8 [DOI] [PubMed] [Google Scholar]
- 59.Huijding J, de Jong PJ. Beyond fear and disgust: the role of (automatic) contamination-related associations in spider phobia. J Behav Ther Exp Psychiatry. 2007;38(2):200–211. doi: 10.1016/j.jbtep.2006.10.009 [DOI] [PubMed] [Google Scholar]
- 60.Olatunji BO, Deacon B. Specificity of disgust sensitivity in the prediction of fear and disgust responding to a brief spider exposure. J Anxiety Disord. 2008;22(2):328–336. doi: 10.1016/j.janxdis.2007.03.007 [DOI] [PubMed] [Google Scholar]
- 61.Matchett G, Davey GC. A test of a disease-avoidance model of animal phobias. Behav Res Ther. 1991;29(1):91–94. doi: 10.1016/S0005-7967(09)80011-9 [DOI] [PubMed] [Google Scholar]
- 62.Davey GC, Bickerstaffe S, MacDonald BA. Experienced disgust causes a negative interpretation bias: a causal role for disgust in anxious psychopathology. Behav Res Ther. 2006;44(10):1375–1384. doi: 10.1016/j.brat.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 63.Mathews A, Mackintosh B. Induced emotional interpretation bias and anxiety. J Abnorm Psychol. 2000;109(4):602. doi: 10.1037/0021-843X.109.4.602 [DOI] [PubMed] [Google Scholar]
- 64.Charash M, McKay D. Attention bias for disgust. J Anxiety Disord. 2002;16(5):529–541. doi: 10.1016/S0887-6185(02)00171-8 [DOI] [PubMed] [Google Scholar]
- 65.Cisler JM, Olatunji BO, Lohr JM, Williams NL. Attentional bias differences between fear and disgust: implications for the role of disgust in disgust-related anxiety disorders. Cogn Emot. 2009;23(4):675–687. doi: 10.1080/02699930802051599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muris P, Mayer B, Huijding J, Konings T. A dirty animal is a scary animal! Effects of disgust-related information on fear beliefs in children. Behav Res Ther. 2008;46(1):137–144. doi: 10.1016/j.brat.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 67.Liu Y-Z, Wang Y-X, Jiang C-L. Inflammation: the common pathway of stress-related diseases. Front Hum Neurosci. 2017;11:316. doi: 10.3389/fnhum.2017.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brydon L, Edwards S, Jia H, et al. Psychological stress activates interleukin-1β gene expression in human mononuclear cells. Brain Behav Immun. 2005;19(6):540–546. doi: 10.1016/j.bbi.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 70.Chen X, Gianferante D, Hanlin L, et al. HPA-axis and inflammatory reactivity to acute stress is related with basal HPA-axis activity. Psychoneuroendocrinology. 2017;78:168–176. doi: 10.1016/j.psyneuen.2017.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang C-J, Stewart JK, Franco RL, et al. LPS-stimulated tumor necrosis factor-alpha and interleukin-6 mRNA and cytokine responses following acute psychological stress. Psychoneuroendocrinology. 2011;36(10):1553–1561. doi: 10.1016/j.psyneuen.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 72.Thoma MV, Gianferante D, Hanlin L, Fiksdal A, Chen X, Rohleder N. Stronger hypothalamus-pituitary-adrenal axis habituation predicts lesser sensitization of inflammatory response to repeated acute stress exposures in healthy young adults. Brain Behav Immun. 2017;61:228–235. doi: 10.1016/j.bbi.2016.11.030 [DOI] [PubMed] [Google Scholar]
- 73.von Känel R, Kudielka BM, Preckel D, Hanebuth D, Fischer JE. Delayed response and lack of habituation in plasma interleukin-6 to acute mental stress in men. Brain Behav Immun. 2006;20(1):40–48. doi: 10.1016/j.bbi.2005.03.013 [DOI] [PubMed] [Google Scholar]
- 74.Boyle NB, Lawton C, Arkbage K, et al. Stress responses to repeated exposure to a combined physical and social evaluative laboratory stressor in young healthy males. Psychoneuroendocrinology. 2016;63:119–127. doi: 10.1016/j.psyneuen.2015.09.025 [DOI] [PubMed] [Google Scholar]
- 75.Wust S, Federenko IS, van Rossum EF, Koper JW, Hellhammer DH. Habituation of cortisol responses to repeated psychosocial stress-further characterization and impact of genetic factors. Psychoneuroendocrinology. 2005;30(2):199–211. doi: 10.1016/j.psyneuen.2004.07.002 [DOI] [PubMed] [Google Scholar]
- 76.Kolmus K, Tavernier J, Gerlo S. β2-Adrenergic receptors in immunity and inflammation: stressing NF-κB. Brain Behav Immun. 2015;45:297–310. doi: 10.1016/j.bbi.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 77.Fitzgerald D, Meade K, McEvoy A, et al. Tumour necrosis factor-a (TNF-a) increases nuclear factor kB (NFkB) activity in and interleukin-8 (IL-8) release from bovine mammary epithelial cells. Vet Immunol Immunopathol. 2007;116(1–2):59–68. doi: 10.1016/j.vetimm.2006.12.008 [DOI] [PubMed] [Google Scholar]
- 78.Renard P, Zachary M-D, Bougelet C, et al. Effects of antioxidant enzyme modulations on interleukin-1-induced nuclear factor kappa B activation. Biochem Pharmacol. 1997;53(2):149–160. doi: 10.1016/S0006-2952(96)00645-4 [DOI] [PubMed] [Google Scholar]
- 79.L-G Ö, Hellström K, Kåver A. One versus five sessions of exposure in the treatment of injection phobia. Behav Ther. 1992;23(2):263–281. doi: 10.1016/S0005-7894(05)80385-5 [DOI] [Google Scholar]
- 80.L-G Ö, Alm T, Brandberg M, Breitholtz E. One vs five sessions of exposure and five sessions of cognitive therapy in the treatment of claustrophobia. Behav Res Ther. 2001;39(2):167–183. doi: 10.1016/S0005-7967(99)00176-X [DOI] [PubMed] [Google Scholar]
- 81.Arntz A, Lavy E. Does stimulus elaboration potentiate exposure in vivo treatment? Two forms of one-session treatment of spider phobia. Behav Cogn Psychother. 1993;21(1):1–12. doi: 10.1017/S0141347300017754 [DOI] [Google Scholar]
- 82.Gotestam K, Hokstad A. One session treatment of spider phobia in a group setting with rotating active exposure. Eur J Psychiatry. 2002;16(3):129–134. [Google Scholar]
- 83.Wolitzky-Taylor KB, Horowitz JD, Powers MB, Telch MJ. Psychological approaches in the treatment of specific phobias: a meta-analysis. Clin Psychol Rev. 2008;28(6):1021–1037. doi: 10.1016/j.cpr.2008.02.007 [DOI] [PubMed] [Google Scholar]