FIGURE 2.
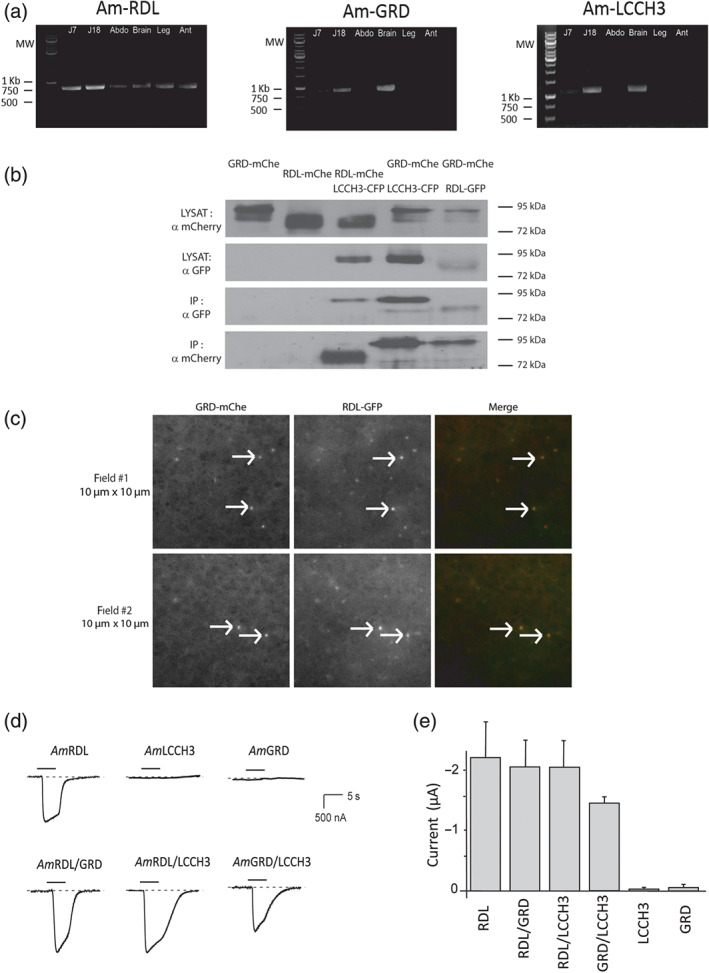
Expression and interaction of AmGABA subunits. (a) RT‐PCR of AmGABA receptor subunits from larvae (J7), nymph (J18), and different parts of adult bodies. RT‐PCR were carried out with the following paired primers: AmRDL: amrdl‐001S and amrdl‐003AS (799pb), AmGRD: amgrd‐001S and amgrd‐004AS (867pb), and AmLCCH3: amlcch3‐001S and amlcch3‐003AS (1143pb). AmRDL transcript is found expressed at all developmental stages and, in the adult honeybee, in all body parts. AmGRD and AmLCCH3 expression are low in larvae, rather high in nymphs, and mostly found in the brain at the adult stage. (Abbreviation: Abdo for abdomen, Ant for antenna). (b) Co‐immunoprecipitation experiments made from HEK293 cells co‐transfected with fluorescently tagged versions of AmGABA receptor subunits. The GFP‐trap system allows the immunoprecipitation of LCCH3‐CFP or RDL‐GFP. Western blot from lysates (input) shows the co‐expression of both proteins in the cells, while anti‐GFP western blot with immunoprecipitation controls both the efficiency and the specificity of the immunoprecipitation. AmLCCH3‐CFP can physically interact with AmGRD‐mChe and AmRDL‐mChe, and AmRDL‐GFP interacts with AmGRD‐mChe. The expected MWs of these tagged proteins are AmGRD‐mChe 85 kDa, AmLCCH3‐CFP 82 kDa, and AmRDL 80 kDa. (c) TIRFM images of oocyte co‐expressing AmGRD‐mCherry and AmRDL‐GFP. Most of the individual protein complex observed on Xenopus oocyte membrane (white arrows) display colocalized spots of mCherry and GFP demonstrating that AmGRD‐mChe and AmRDL‐GFP are able to co‐assemble as a single GABA receptor. (d) Representative current traces obtained by applying 100‐μM GABA to Xenopus oocytes with different AmGABA receptor subunits expressed alone or in combination. Only AmRDL is able to form a functional homopentamer sensitive to GABA application. In contrast, all possible heteromer combinations produce a GABA‐sensitive channel. (e) Averaged values of AmGABA receptor current amplitudes evoked by 100‐μM GABA application (n = 10). The amplitudes of the currents elicited by the stimulation of AmGRD/LCCH3 are slightly smaller than those elicited by the activation of AmRDL‐containing heteromers
