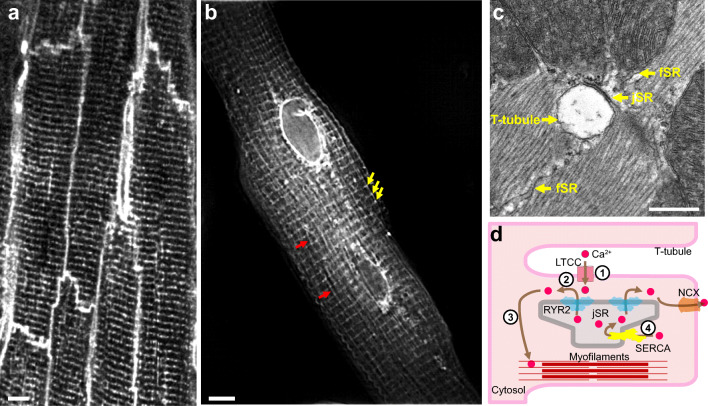
Fig. 1.
Anatomy of cardiac dyads. (A) T-tubule network of mature adult cardiomyocytes. The mouse heart was perfused with MM-4-64, which stains the sarcolemma, and optically sectioned and imaged using a confocal microscope. Bar = 5 μm. (B) Total sarcoplasmic reticulum (SR) structure of an adult cardiomyocyte. The dissociated adult mouse cardiomyocyte was stained using Fluo5N and visualized by structured illumination microscopy. Note the discrete junctional SR (jSR; yellow arrows) and the diffusible free SR (fSR; red arrows) and the much brighter perinuclear envelopes. Bar = 5 μm. (C) Longitudinal section of mouse left ventricular myocyte in a thin section transmission electron micrograph. A T-tubule, shown in cross section, is separated from pancake-shaped jSR cisternae by a narrow (~12 nm) junctional gap. Electron dense “foot” structures corresponding to the cytoplasmic domain of RYR2 protrude into the gap. fSR is contiguous with jSR. Bar = 0.5 μm. (D) Cartoon showing the process of EC coupling. At dyadic junctions, L-type Ca2+ channels (LTCCs) in T-tubules are coupled with RYR2 Ca2+ release channels in jSR. (1) The action potential depolarizes T-tubule membranes, opening LTCCs. (2) Extracellular Ca2+ enters through LTCCs into dyadic clefts, resulting in Ca2+-induced Ca2+ release through RYR2. (3) The resulting increase in [Ca2+]i causes sarcomere contraction. (4) [Ca2+]i is returned to basal levels by SERCA2a, which actively pumps Ca2+ back into SR, and by extrusion through the Na+/Ca2+ exchanger, NCX