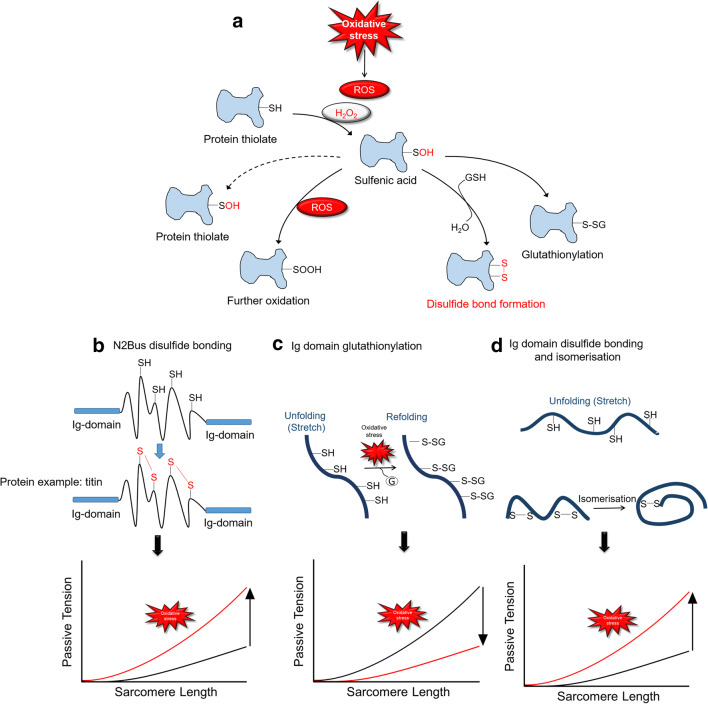
Fig. 3.
Thiol modifications of proteins and mechanisms of titin-based passive tension modulation by oxidative stress–induced titin modifications. A Formation of sulfenic acid from the reaction of H2O2 with protein thiolates. This formation leads to different protein modifications. In proteins without a second sulfhydryl, the sulfenic acid (–SOH) may be stabilized or will generate oxidized sulfinic (–SOOH) and sulfonic acid derivatives due to its reaction with ROS. Otherwise, a disulfide bond can form between the two sulfur atoms (–S–S–). Lastly, the sulfenated cysteinyl residue can react with glutathione (GSH), leading to a mixed disulfide. B Formation of intramolecular disulfide bonds within the titin-N2Bus when exposed to oxidative stress, which then increases titin-based stiffness in cardiomyocytes. C Ig domain unfolding due to sarcomere stretching causes exposure of hidden (“cryptic”) cysteines in Ig domains, which can become S-glutathionylated under oxidative conditions. This modification prevents Ig domain refolding, resulting in decreased titin-based stiffness. D Isomerization of disulfide bonds of the cysteine triad in titin Ig domains can occur under oxidative conditions. This modification leads to increased titin based stiffness