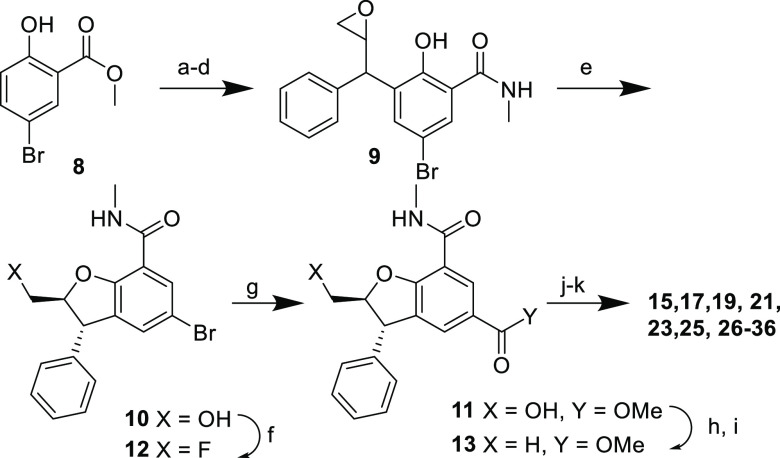
Scheme 1. Synthesis of Functionalized DBFs.
Reagents and conditions: (a) Cinnamyl chloride, K2CO3, KI, acetone, reflux, 100%; (b) N,N-dimethylaniline, reflux; (c) MeNH2, THF, room temperature, 56% (two steps); (d) m-CPBA, CH2Cl2, 0 °C, 98%; (e) KOH, DMSO/H2O, 0 °C, 67%; (f) Deoxofluor, CH2Cl2, 0–40 °C, 71%; (g) Pd(OAc)2, Xantphos, CO, MeOH, DMF, 70 °C, 49%; (h) I2, PPh3, imidazole, CH2Cl2, rt, 79%; (i) NEt3, Pd/C, H2 (1 atm), MeOH, rt, 56%; (j) LiOH or NaOH, MeOH, THF, H2O, rt, >95%; (k) RNH2, HATU, NEt3, rt, 13–92%.