Fig. 1. CAR structure and mechanisms for response to soluble ligands.
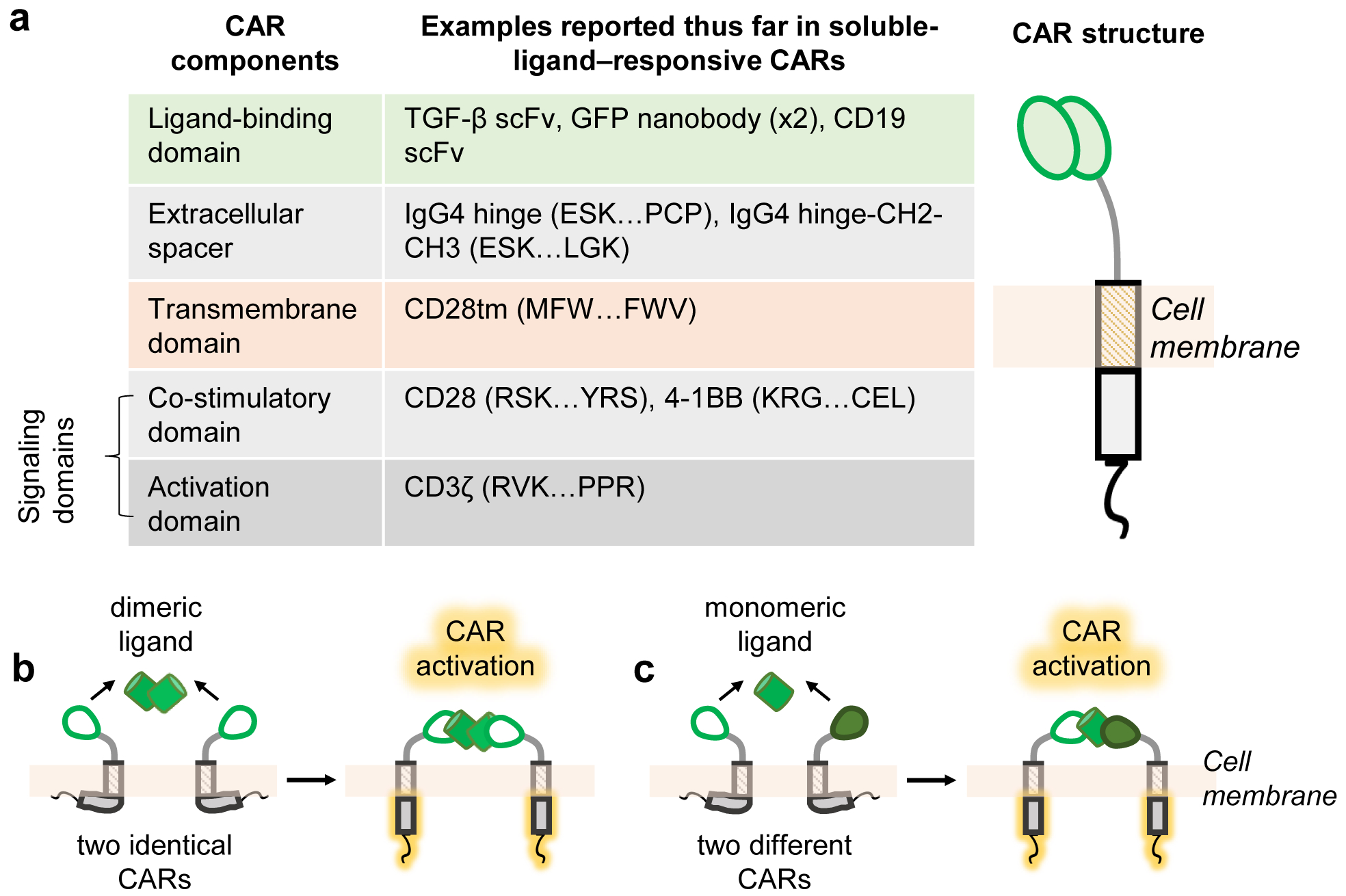
a, A CAR presents an extracellular ligand-binding domain linked via an extracellular spacer and transmembrane domain to intracellular signaling domains. The indicated specific domains have been shown to function in the context of responding to natural and synthetic soluble ligands. For common domains sourced from native proteins, the specific amino acid stretches we have used are demarcated by the first 3 amino acids and last 3 amino acids6,23. The full amino acid sequence of each native protein can be obtained from public databases such as NCBI and Uniprot. b, CARs signal in response to soluble ligand if the cognate ligand contains two binding epitopes that can cause CAR dimerization. c, For ligands of interest that do not contain multiple binding epitopes, ligand responsiveness can be conferred by expressing two CARs capable of simultaneously binding separate epitopes on the same ligand. In b-c, the proposed models shown assume that CAR cytoplasmic tails interact with the plasma membrane in the inactive state, based on the membrane association observed for CD28 and CD3ζ cytoplasmic domains in their native contexts32–34.
