Fig. 5. Degranulation in response to soluble and immobilized target ligand.
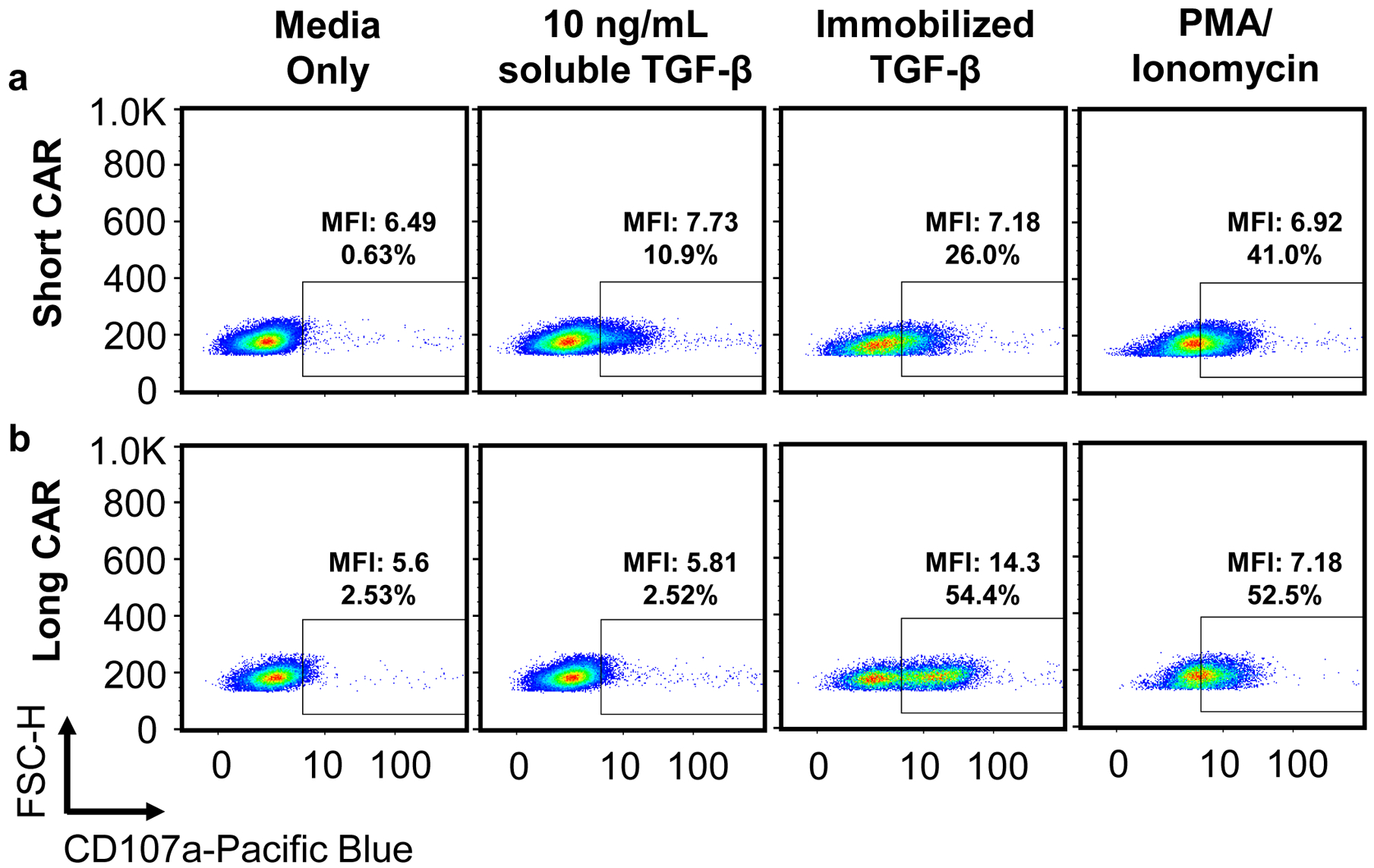
T cells expressing a TGF-β CAR with an IgG4 hinge spacer (a, short CAR) and IgG4-CH2-CH3 spacer (b, long CAR) were exposed to media alone, 10 ng/mL soluble TGF-β, immobilized TGF-β, or 50 ng/mL PMA and 1 μM ionomycin. TGF-β was immobilized in cell-culture wells by covering the well with 100 ng/mL TGF-β at room temperature for 30 min, washing once with PBS, then allowing the well to dry. Analysis of degranulation according to steps 52–57 showed that when targeting TGF-β immobilized on the bottom of culture wells, only the long-CAR cell line exhibits a distinct CD107ahi population consistent with that of a full degranulation response, perhaps because only the long CAR provides appropriate T-cell–to–target spacing. In contrast, for both cell lines, stimulation with either soluble TGF-β or soluble PMA/ionomycin resulted in a broadening of the CD107a expression distribution into the CD107a+ gate, but no distinct CD107ahi degranulated population. Scatter plots are representative of n = 3 samples.
