Figure 1.
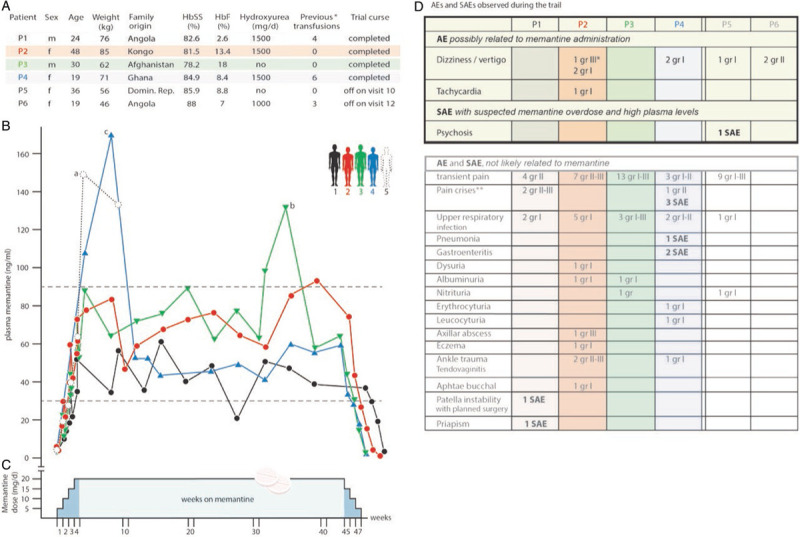
Study design, information on the patients and plasma memantine pharmacokinetics. A. Demographics of the SCD patients and characteristics at enrolment. Sex, age, weight and family origin as well as the corresponding percentage of HbS and HbF measured during the screening period of patients P1-6 are shown. All patients had the HbSS phenotype. ∗number of previous transfusions that applied 9 to 12 months before enrolment. B. Plasma memantine pharmacokinetics during the study periods. The analysis of the memantine concentration in the plasma of the patients revealed a good compliance during the trial with the following exceptions: overdosing by the patient P5 (a) led to psychosis (SAE) and the trial was terminated. Overdosing by P3 (b) and P4 (c) caused reversible increase in plasma memantine levels without discontinuation. Note that the memantine levels found in P6 who stopped the trail at week 5 was within the normal range (not shown). C. Schematic representation of the trial periods. The study was divided into five periods as described in the Supplementary Data. D. Adverse Effects (AE) and Severe Adverse Effects (SAE). The upper part of the table shows the AE and SAE that were attributed to memantine intake, while the lower part reflects the AE and SAE that were considered as not likely related to memantine. ∗Dizziness/vertigo led to a transient dose reduction (10 mg per day for 10 days) that was increased again to 20 mg after cessation of this symptom. ∗∗A pain crisis was defined as ongoing pain lasting for longer than 4 hours (shorter pain periods are defined as “transient pain”) and requiring the application of opiates or treatment at the out patients clinic or emergency department for less than 12 hours.
