Fig. 1.
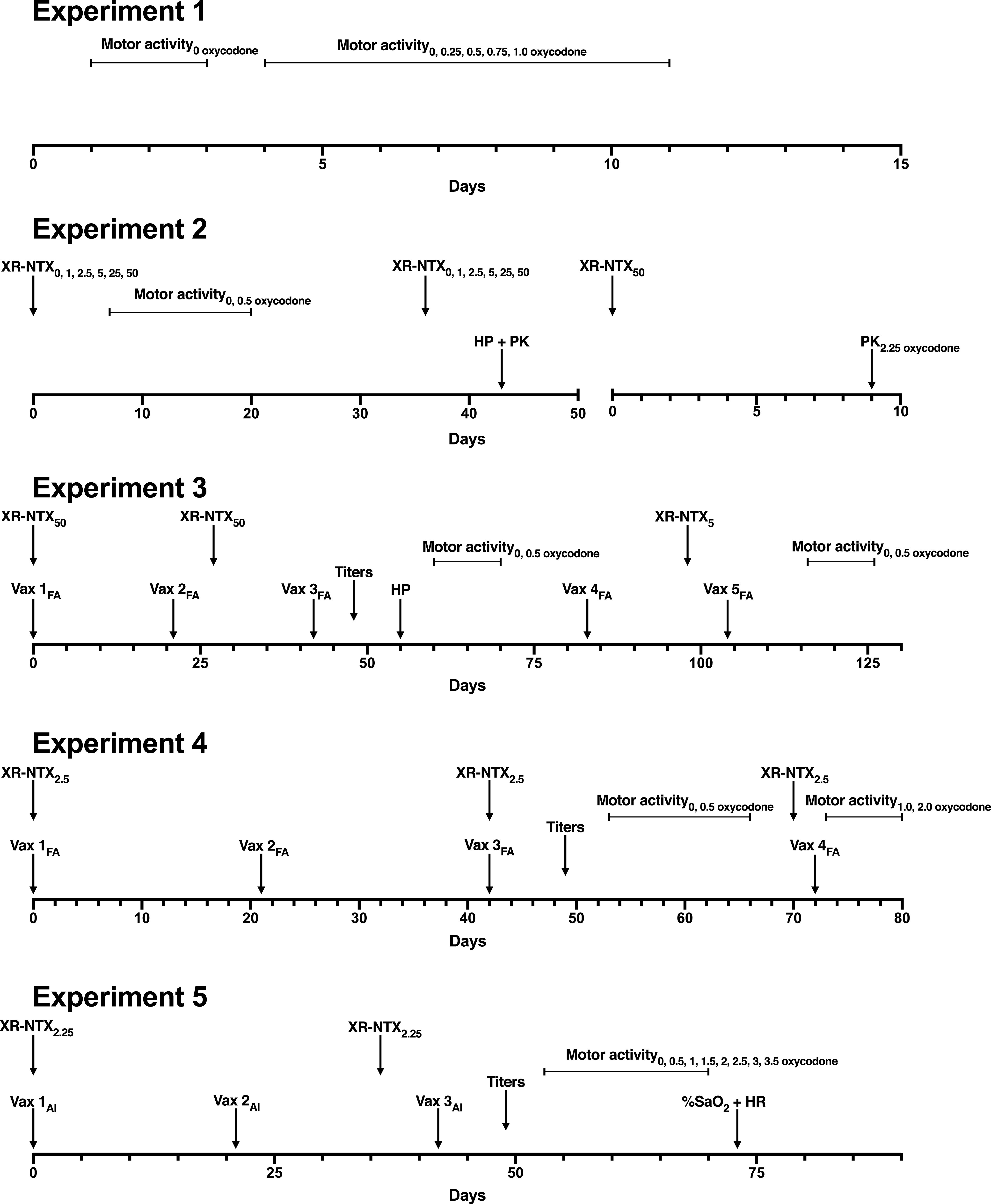
Design overview of Experiments 1 through 5. XR-NTX doses were given intramuscularly with subscripts denoting milligrams per kilogram dose received. Sal, saline administration. The gap in the x-axis in Experiment 2 depicts a separate cohort of animals. HP + PK refers to antinociceptive testing after subcutaneous administration of 2.25 mg/kg oxycodone on the hot-plate (HP) set to 54°C, which was followed by blood and brain collection, whereas PK2.25 refers to the pharmacokinetics (PK) study. For Experiments 3 through 5, vaccinations (Vax) are shown as Vax # with # denoting order of vaccination and subscripts denoting Freund’s Adjuvant (FA) or aluminum (Al). Blood draws for titer analyses are shown as “Titers.” Motor activity sessions are shown from start to finish, with subscripts denoting subcutaneous oxycodone doses. %SaO2 + HR refer to oximetry and bradycardia (HR, heart rate) testing in Experiment 5.
