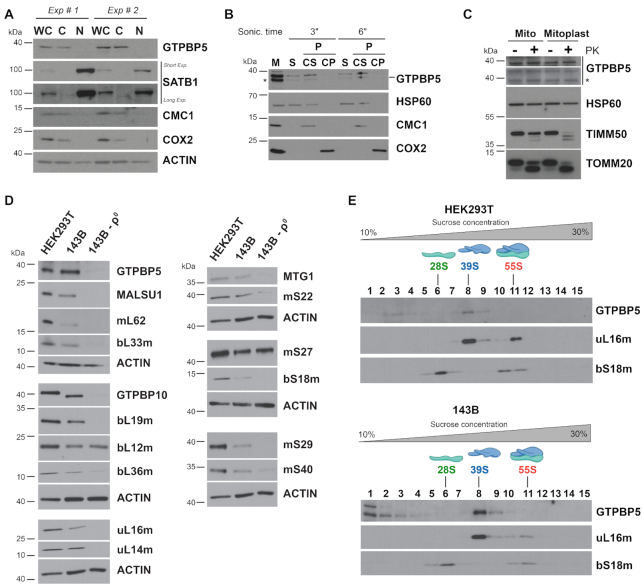
Figure 1.
GTPBP5 is a mitochondrial protein that sediments with the mitoribosome large subunit (mtLSU). (A) Immunoblot analyses of GTPBP5 levels in HEK293T whole-cell lysate (WCL), cytoplasmic (C) and nuclear (N) fractions. Antibodies against mitochondrial proteins CMC1 and COX2 and nuclear protein SATB1 were used as controls. (B) Mitochondria (M) isolated from HEK293T cells were first fractionated into soluble (S) and membrane-bound (P) proteins by brief sonication and centrifugation. The pellet was submitted to alkaline carbonate extraction (pH: 11.5) to allow the separation of the extrinsic proteins present in the supernatant (CS) from the intrinsic proteins in the pellet (CP). Equivalent volumes of each fraction were analyzed by immunoblotting using antibodies against GTPBP5, the matrix-soluble protein HSP60, the extrinsic membrane-associated protein CMC1, and the inner membrane intrinsic protein COX2. In the anti-GTPBP5 immunoblot, the asterisks mark the GTPBP5 protein. (C) Proteinase K protection assay in mitochondria (Mt) and mitoplasts (Mp) prepared by hypotonic swelling of mitochondria. The samples were analyzed by immunoblotting using antibodies against GTPBP5, the matrix protein HSP60, the inner membrane protein TIM50, and the outer membrane protein TOM20. In the anti-GTPBP5 immunoblot, the asterisk marks the GTPBP5 protein. (D) Immunoblot analyses of the steady-state levels of mitoribosome LSU, SSU proteins and assembly factors in whole-cell extracts from HEK293T, 143B and 143B-206 rho0 (ρ0) cells. ACTIN was used as a loading control. (E) Sucrose gradient sedimentation analyses of GTPBP5 and mitoribosomal proteins in mitochondrial extracts from wild-type HEK293T or 143B mitochondria prepared in the presence of the 20 mM MgCl2. The fractions were analyzed by immunoblotting using antibodies against the indicated proteins.