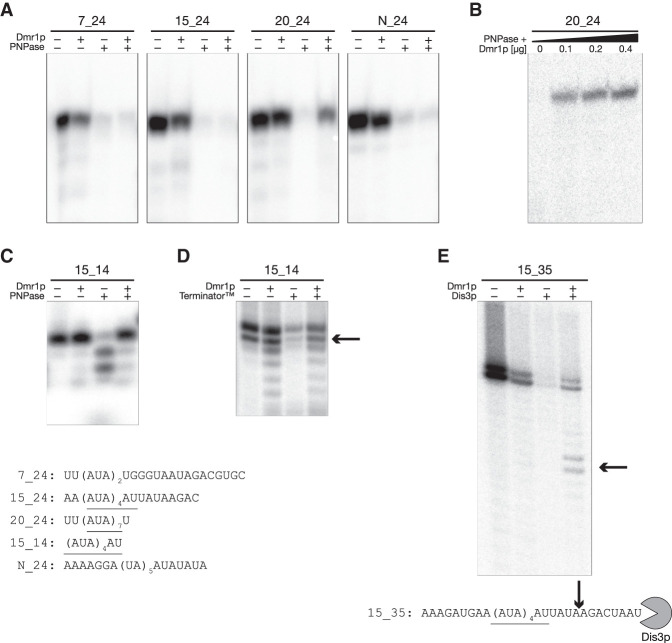
FIGURE 3.
Binding by Dmr1p protects specifically recognized RNA fragments from exoribonuclease degradation. (A) Binding by Dmr1p protects the 24 nt fragment containing seven AUA repeats (20_24) from degradation by 3′–5′ exoribonuclease—polynucleotide phosphorylase. An amount of 1.2 µg of Dmr1-His6 fusion protein was preincubated with the radiolabeled RNA substrate prior to the addition of recombinant Synechocystis sp. PNPase. (B) Increasing the amount of Dmr1p increases the fraction of radiolabeled RNA (20_24) protected from degradation by PNPase. Total amount of recombinant Dmr1-His6 fusion protein is indicated for each lane. (C,D) Binding by Dmr1p protects the 14 nt radiolabeled RNA corresponding to the (AUA)4AU recognition motif (15_14) from degradation by 3′–5′ exoribonuclease—PNPase (C) and 5′–3′ exoribonuclease—Terminator (D). The radiolabeled substrate was treated with 5′ polyphosphatase prior to the assay. The monophosphorylated substrate that is susceptible to the 5′–3′ exoribonucleolytic activity of Terminator is indicated with an arrow. An amount of 1.2 µg of Dmr1-His6 fusion protein was preincubated with the radiolabeled RNA substrate prior to the addition of the nucleases as described in Materials and Methods. (E) Dmr1p bound to the 35 nt substrate stops degradation by recombinant Dis3 3′–5′ exoribonuclease at a site 4–5 nt downstream from the (AUA)4AU recognition motif (marked by arrow). Sequences of fragments used in each assay are shown below respective gels, with the AUA trinucleotide repeats underlined.