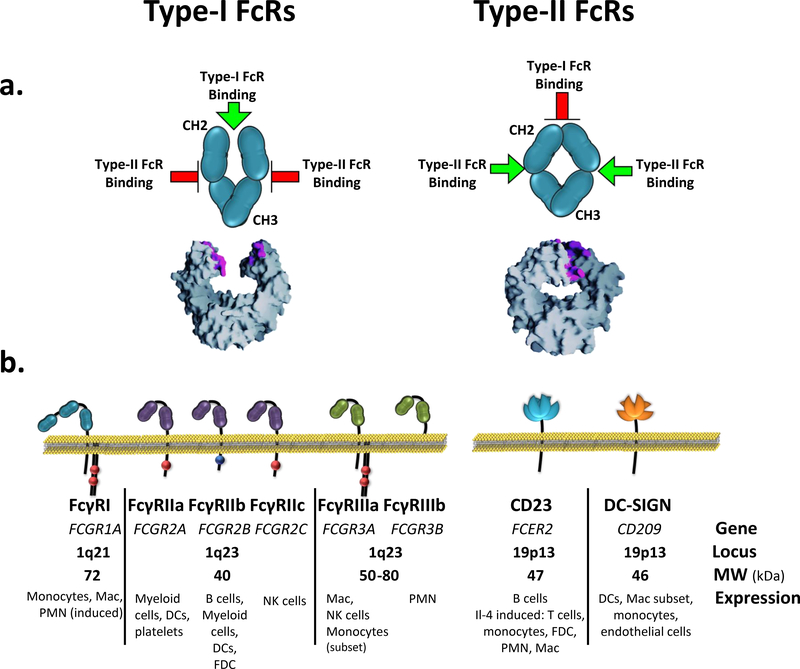
Figure 1. Reciprocal engagement of Type-I and Type-II receptors by IgG Fc domain.
a) The Fc domain alternates between open and closed conformations depending on the sialylation status of the Fc glycan. Non-sialylated Fc adopts an open conformation capable of binding Type-I receptors near the hinge-proximal surface, whereas the binding site for Type-II receptors remains inaccessible. Upon sialic acid conjugation, the Fc acquires a closed conformation that occludes the Type-I receptor binding site and reveals a binding site for Type-II receptors. (PDB file:3AVE, for non-sialylated Fc structure). b) Schematic and expression profile of the human Type-I and Type-II receptors known to bind the Fc domain of IgG. Type-I and Type-II receptors form part of the Ig-family and C-type lectin receptors, respectively. Mac, macrophage; PMN, polymorphonuclear leukocytes; NK, natural killer; FDC, follicular dendritic cell; DC, dendritic cell.