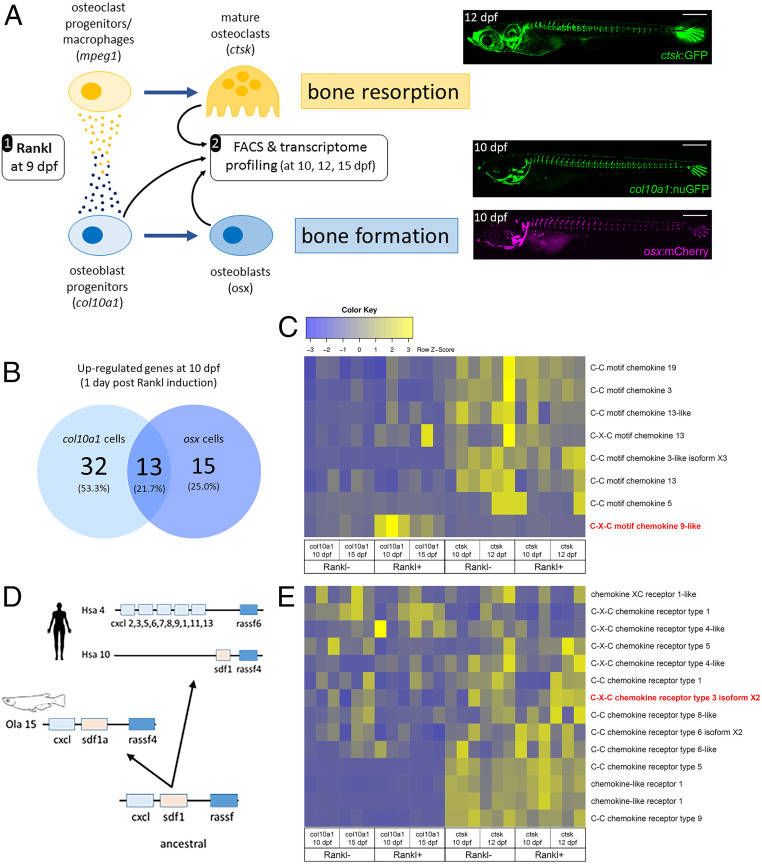
Fig. 1.
Transcriptome profiling of bone cells in a medaka osteoporosis model. (A) Experimental design: Medaka transgenic for rankl:HSE:cfp and carrying a reporter for macrophages (e.g., mpeg1:mCherry), osteoclasts (e.g., ctsk:GFP; Top Right), osteoblast progenitors (e.g., col10a1:nuGFP; Middle Right), and premature osteoblasts (e.g., osx:mCherry; Bottom Right) were heat-shocked at 9 dpf. This induced rankl expression, which in turn activated osteoblast (col10a1) and osteoclast (mpeg1) progenitor cells. Signaling between progenitors, as indicated by small circles, was suggested to trigger macrophage differentiation into osteoclasts (ctsk) in the vertebral column (image) and increased bone resorption, as well as differentiation of osteoblasts (osx) for bone formation. Larvae were dissociated to obtain col10a1 (at 10 and 15 dpf), osx (10 dpf), and ctsk (10 and 12 dpf) cells by FACS. Rankl nontransgenic reporter larvae after heat shock were used as controls. For details, see ref. 21. (B) Venn diagram showing number of significantly up-regulated genes in osteoblast progenitors and osteoblasts, at 10 dpf, 1 d after Rankl induction. (C) Heat map showing RNAseq-based expression levels of chemokine ligands in triplicates of osteoblast progenitors (col10a1, at 10 and 15 dpf) and osteoclasts (ctsk, at 10 and 12 dpf) without and with Rankl induction at 9 dpf. Note Rankl-induced up-regulation of cxcl9l (red) exclusively in col10a1 cells. (D) Inferred evolutionary history of C-X-C chemokine ligands. Deduced genomic organization of cxcl9l and neighboring genes on ancestral, medaka, and human chromosomes. (E) Expression levels of selected chemokine receptors. Cxcr3.2 is highlighted in red.