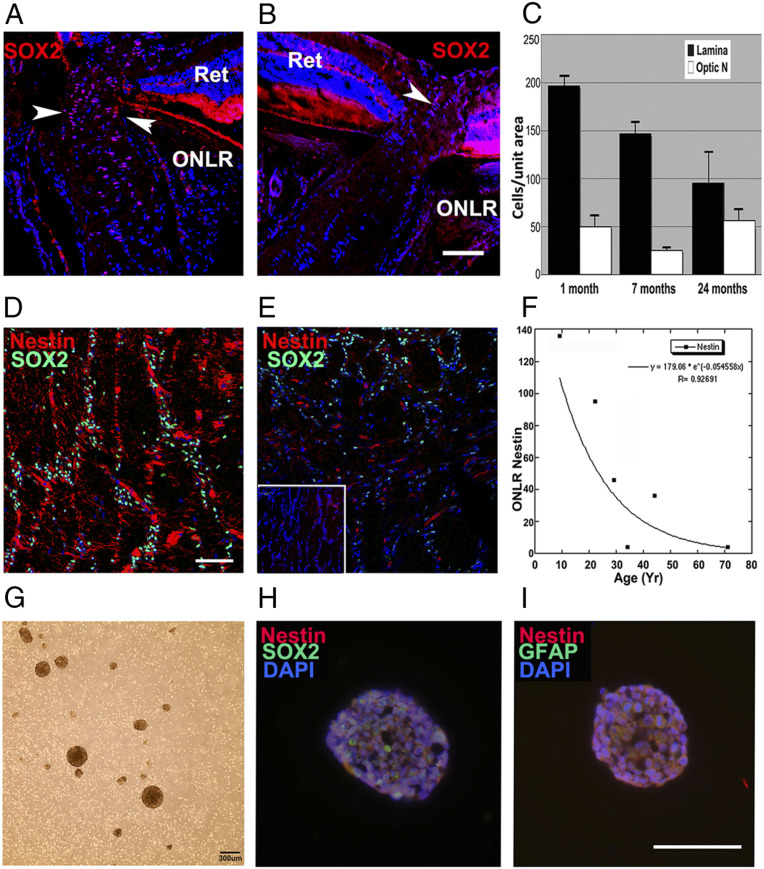
Fig. 5.
Age-related NPC depletion in mouse and human ONLR and neurosphere development from human donors. (A) SOX2 nuclear expression (red) in a 6-mo mouse ONLR. Nests of SOX2(+) nuclei are present in the ONLR and anterior ON (arrowheads). (B) SOX2 nuclear expression in a 2 y/o mouse ONLR. There are fewer SOX2(+) nuclei (arrowhead), and SOX2(+) signal intensity is reduced. (Scale bar: 50 μm.) (C) SOX2 nuclear quantification in mouse ONLR and ON during aging. SOX2 nuclei declined 73% from 1 mo to 2 y (black bars) while SOX2 nuclear numbers in distal ON (white bars) remain relatively constant during this time (n = 6 nerves/group; data ± SEM). (D and E) Analysis of human ONLR-nestin and -SOX2 expression, with SOX2 immunostaining (green) and Nestin (red). (D) Forty y/o ONLR. Filamentous nestin expression is present, with numerous SOX2(+) nuclei. (Scale bar: 100 μm.) (E) Fifty y/o ONLR. There is reduced filamentous nestin expression, with remaining nestin present almost exclusively in the vasculature, and greatly reduced SOX2(+) nuclei. Inset: 71 y/o ONLR. The elderly ONLR reveals both a total lack of filamentous nestin and an absence of SOX2(+) nuclei. (F) Densitometric analysis of age-related human ONLR nestin expression. Nestin expression declines linearly from 9 y/o to 45 y/o, with severe loss in individuals >50 y of age. Donor history is shown in SI Appendix, Table S1A. (G) Human ONLR neurosphere formation (33 y/o donor). (H) SOX2 (green)/nestin (red) Immunolabeling of human neurosphere from culture shown in G. Individual cells express both SOX2 and nestin. (I) Nestin (red)/GFAP (green) expression in human neurosphere. Nestin and GFAP colocalize in individual cells. (Scale bar: 100 μm.)