Figure 1.
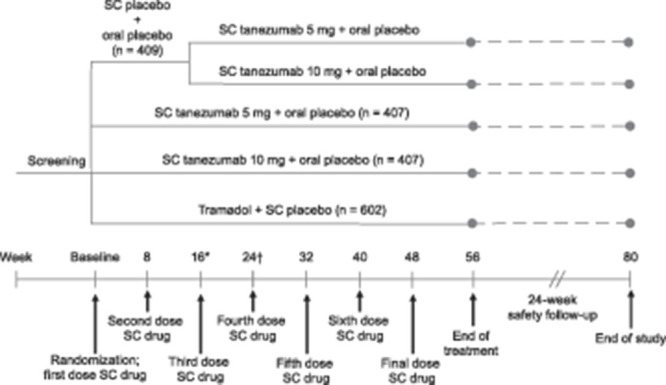
Study design. Subcutaneous (SC) treatment was administered every 8 weeks and oral treatment was administered daily. At week 16, patients in the placebo arm were transitioned in a blinded 1:1 ratio to tanezumab 5 or 10 mg. Oral treatment was initiated at 100 mg/day and could be adjusted in 100 mg increments at weeks 1, 2, 3, and 4 to a maximum of 300 mg/day. The dose of oral medication remained stable from weeks 5 through 56 (titration of oral medication was allowed in Europe after week 16). Scheduled in-clinic visits occurred at baseline, and weeks 2, 4, 8, 16, 24, 32, 40, 48, 56, 64, and 80. Scheduled phone contact with the subject occurred at weeks 1, 3, 12, 20, 28, 36, 44, 52, 60, 68, 72, and 76. *Before receiving treatment at week 16, patients must have had a ≥30% reduction from baseline in average low back pain intensity (LBPI) score at week 16 and a ≥15% reduction from baseline in mean weekly LBPI score at any week from weeks 1 through 15 to continue the study. †Before receiving treatment at week 32, patients must have had a ≥30% reduction from baseline in LBPI score to continue the study. Numbers based on the safety population.
