Figure 3.
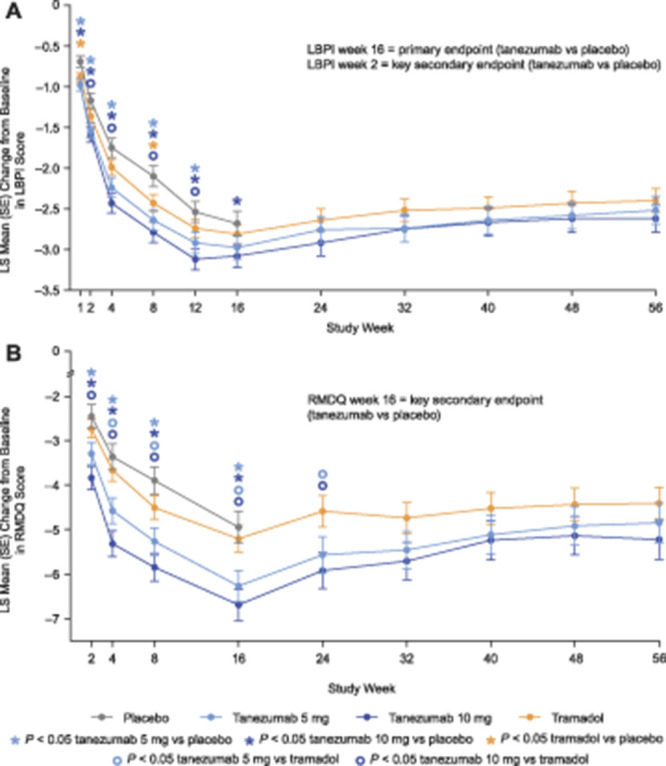
Change in LBPI (A) and RMDQ (B) scores from baseline to week 56. Change in low back pain intensity (LBPI) score at week 16 (tanezumab vs placebo) was the primary efficacy endpoint. Change in LBPI score at week 2 (tanezumab vs placebo) was a key secondary endpoint. Change in Roland Morris Disability Questionnaire (RMDQ) score at week 16 (tanezumab vs placebo) was a key secondary endpoint. Comparisons of tanezumab to placebo at other time points, and comparisons of tramadol to other treatment groups at any time point, were secondary endpoints. The primary and key secondary endpoint analyses were adjusted for multiple comparisons; other secondary analyses were not adjusted for multiplicity. See text for details. LS, least squares; SE, standard error.
