Table 1.
Characteristics of 122 patients with UC
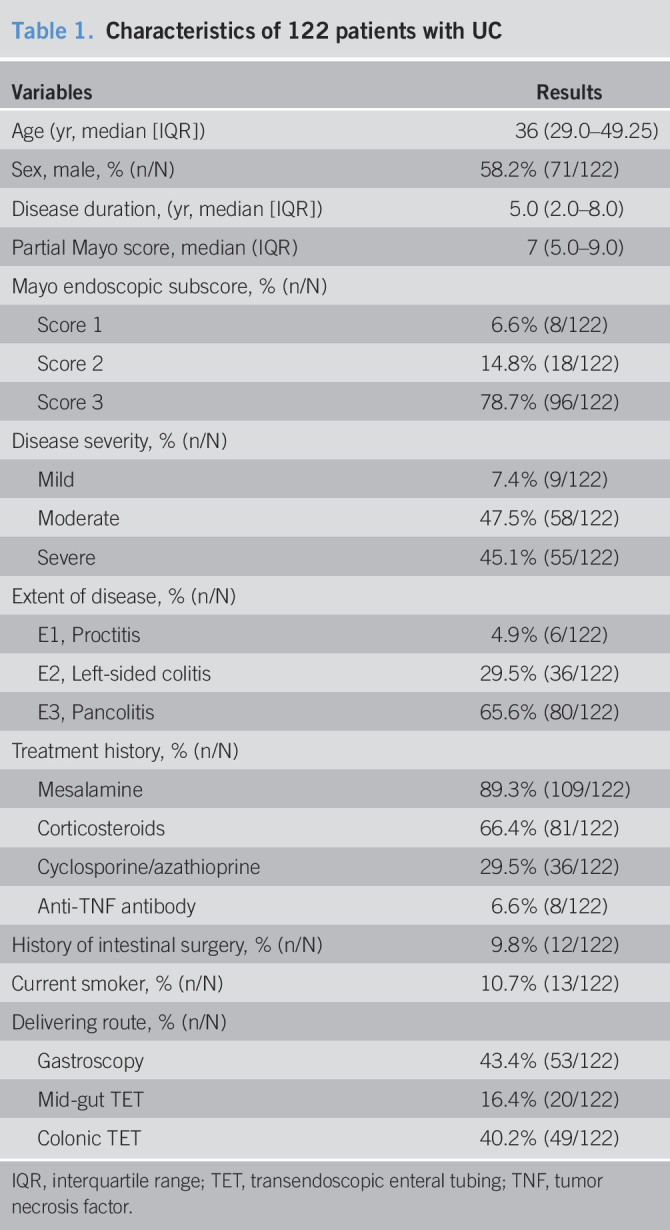
| Variables | Results |
| Age (yr, median [IQR]) | 36 (29.0–49.25) |
| Sex, male, % (n/N) | 58.2% (71/122) |
| Disease duration, (yr, median [IQR]) | 5.0 (2.0–8.0) |
| Partial Mayo score, median (IQR) | 7 (5.0–9.0) |
| Mayo endoscopic subscore, % (n/N) | |
| Score 1 | 6.6% (8/122) |
| Score 2 | 14.8% (18/122) |
| Score 3 | 78.7% (96/122) |
| Disease severity, % (n/N) | |
| Mild | 7.4% (9/122) |
| Moderate | 47.5% (58/122) |
| Severe | 45.1% (55/122) |
| Extent of disease, % (n/N) | |
| E1, Proctitis | 4.9% (6/122) |
| E2, Left-sided colitis | 29.5% (36/122) |
| E3, Pancolitis | 65.6% (80/122) |
| Treatment history, % (n/N) | |
| Mesalamine | 89.3% (109/122) |
| Corticosteroids | 66.4% (81/122) |
| Cyclosporine/azathioprine | 29.5% (36/122) |
| Anti-TNF antibody | 6.6% (8/122) |
| History of intestinal surgery, % (n/N) | 9.8% (12/122) |
| Current smoker, % (n/N) | 10.7% (13/122) |
| Delivering route, % (n/N) | |
| Gastroscopy | 43.4% (53/122) |
| Mid-gut TET | 16.4% (20/122) |
| Colonic TET | 40.2% (49/122) |
IQR, interquartile range; TET, transendoscopic enteral tubing; TNF, tumor necrosis factor.
