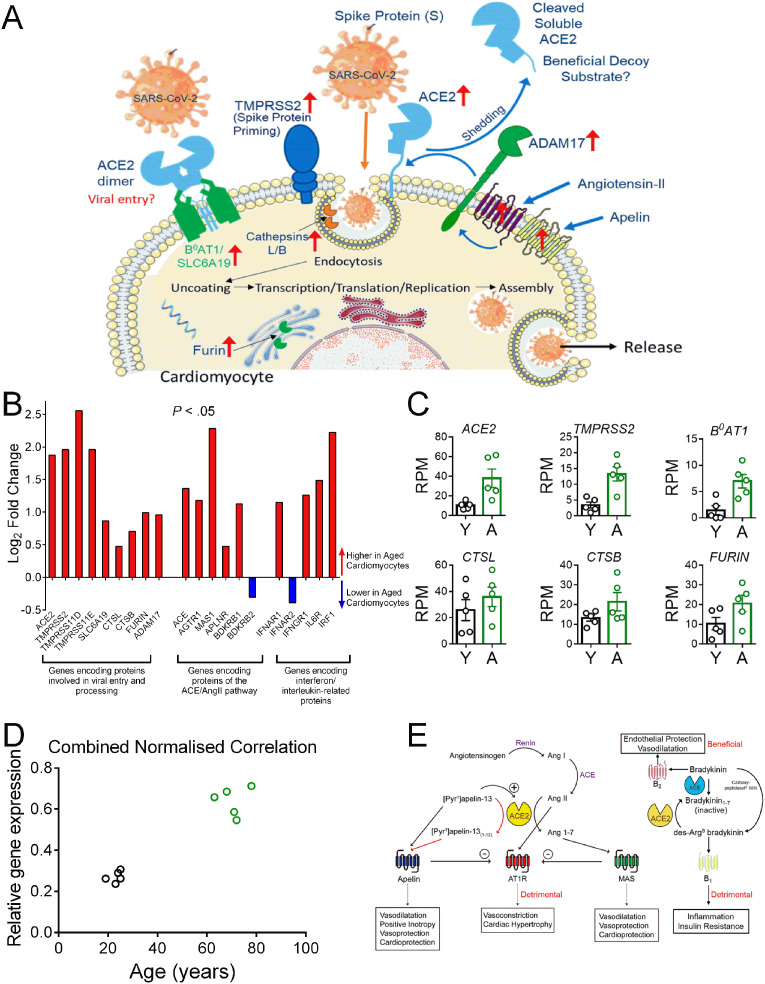
Fig. 1.
Genes Encoding Proteins Mediating Viral Entry are Upregulated with Age. See recent review [4] for a comprehensive list of references supporting the concepts outlined in this letter.
[A] Schematic diagram of the key proteins predicted from RNASeq data to be expressed by human cardiomyocytes. We propose SARS-CoV-2 binds initially to ACE2 (with the ACE2/B0AT1 complex as a potential second entry site). TMPRSS2 priming of the spike protein S1, together with further protease activation by cathepsins B and L, facilitates viral cell entry and internalization by endocytosis. Furin may also have a role in this process. Internalization of the virus with ACE2 inhibits ACE2 carboxypeptidase activity that normally hydrolyses Ang-II, apelin and des-Arg9-bradykinin. ADAM17, present on the cell surface, cleaves ACE2 to a soluble form that circulates in the plasma and could act as a decoy substrate for the virus. Levels of ADAM17 may be regulated by Ang-II and apelin acting via their respective G protein-coupled receptors [4]. ↑ - indicates genes with increased expression in aged cardiomyocytes vs young.
[B] Log2 fold change for expressed genes encoding SARS-CoV-2 entry proteins, peptide receptors in ACE/ACE2 pathway and IL-6 and interferon receptors in aged cardiomyocytes. A Chi Squared analysis for the selected gene panel shown in [B] showed significant enrichment for differentially expressed genes compared with the RNASeq data set as an entirety (P < 0.05, 95% confidence level).
[C] Scatter dot plot showing individual data points (with mean ± S.E.M) of key genes with higher expression in young (Y) verses aged (A) cardiomyocytes. Aged cardiomyocytes were double positive for ACE2 and TMPRSS2, critical for viral entry.
[D] Cardiomyocyte expression levels of the gene panel shown in [C] correlates positively with age. For each of the six targets presented in [C], the mean relative gene expression was calculated, normalized to the sample with the highest expression in RPM (non-parametric Spearman correlation, two-tailed, r = 0.86, P = 0.0025).
[E] Schematic diagram of genes encoding GPCRs that show higher expression in aged human cardiomyocytes compared with young. Activation of the renin-angiotensin system, with overproduction of Ang-II, contributes to acute respiratory distress syndrome following infection by coronoviruses. The Ang-II synthetic enzyme ACE and the ANG-II cognate receptor gene AGTR1 increased with age, together with B1 receptor gene, BDKBR1. This bradykinin receptor is selectively activated by des-Arg9-bradykinin (normally inactiveated by ACE2), representing a second deleterious pathway. Both pathways could be blocked with clinically approved drugs (ACE inhibitors, AT1 receptor antagonists and the B2 receptor antagonist Icatibant) for secondary treatment in pateints with COVID-19. Internalization of ACE2 by the virus is predicted to increase Ang II levels but reduce those of Ang1–7 but the gene encoding the proposed Ang1–7 receptor, MAS, was still detected in aged cardiomyocytes, as was the apelin receptor. The beneficial potent positive inotropic action of apelin in the heart as well as its anti-thrombotic and anti-diabetic properties, suggests its receptor is a promising therapeutic target for administered apelin and this strategy is currently under clinical investigation.