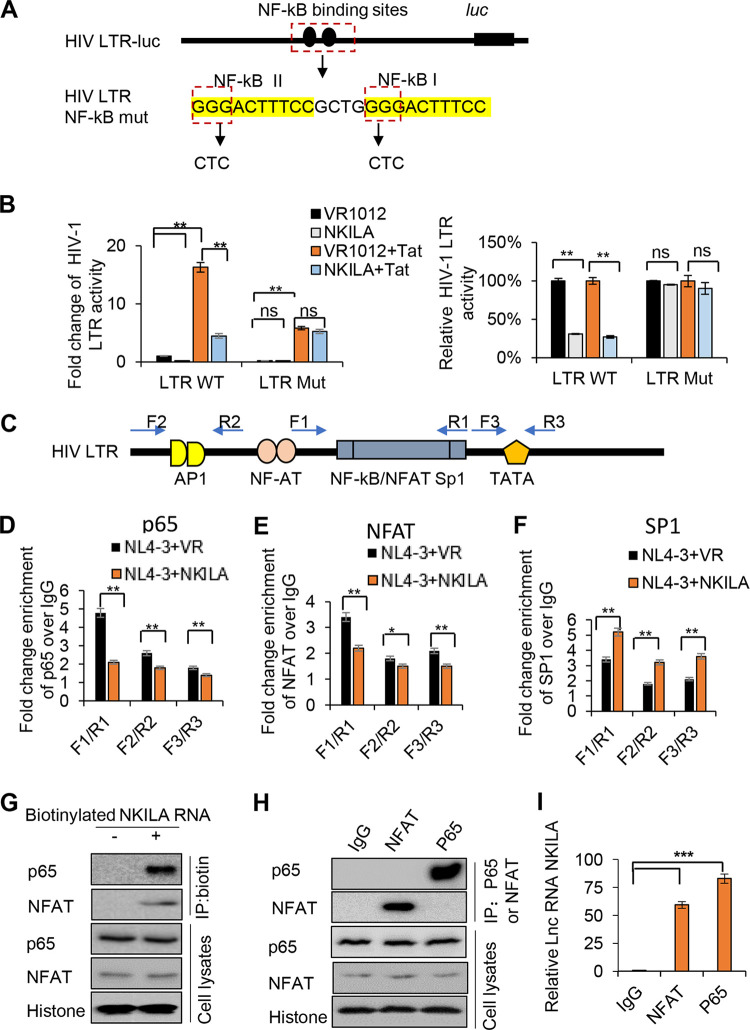
FIG 5.
NKILA directly inhibits NF-κB-mediated HIV-1 transcription by obstructing p65 recruitment to the LTR promoter. (A) Representation of the HIV-LTR with mutations at NF-κB binding sites. (B) The effect of NKILA on the HIV-1 LTR promoter is specifically mediated by NF-κB binding sites. pHIV-1 5′ LTR WT or pHIV-1 5′ LTR mutant plasmids were cotransfected with NKILA or negative-control vector, and cells were harvested for a reporter assay 48 h posttransfection. (C) Schematic of the HIV-1 LTR primers (F1/R1, F2/R2, and F3/R3) used in the ChIP assays. (D) p65 recruitment to the LTR promoter at the NF-κB-SP1 enhancer region was disrupted by NKILA in HIV-1 NL4-3 virus-infected Jurkat cells. Jurkat cells transfected with NKILA or negative-control vector for 24 h were infected with HIV-1 NL4-3 virus for another 5 days. Fixed and isolated chromatin from HIV-1-infected Jurkat cells was immunoprecipitated with anti-p65 antibody or IgG as the negative control and was analyzed by qRT-PCR with the indicated primers spanning three different regions in the LTR promoter. The effect of NKILA on the recruitment of NFAT (E) or SP1 (F) to HIV-1 LTR. The experimental procedure is the same as for panel D. Fixed and isolated chromatin was immunoprecipitated with anti-NFAT or SP1 antibody or IgG as the negative control and then was analyzed as described for panel D. (G) The interaction of p65 and NFAT proteins with NKILA according to RNA pulldown assay. (H) NKILA interacted with NFAT and p65 proteins according to the co-IP assay. Jurkat cells were immunoprecipitated with anti-NFAT- or p65 antibody-conjugated agarose beads. NFAT and P65 proteins were detected by immunoblotting analysis. (I) The relative binding ability between NFAT or P65 with NKILA. The binding between NKILA and IgG was set as 1. **, P < 0.01; ***, P < 0.001.