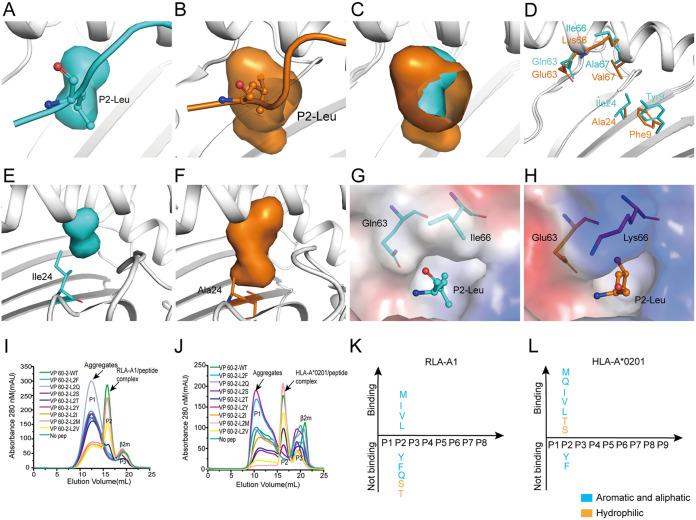
FIG 3.
The small and hydrophobic B pocket of RLA-A1. (A) Pocket B of RLA-A1 is shown as cyan cavities. P2-Leu of VP60-2 is shown as cyan sticks and spheres. The backbone of RLA-A1 is shown as a cartoon in white. (B) The B pocket of HLA-A*0201 is shown as orange cavities. P2-Leu of the peptide is shown as orange sticks and spheres. The backbone of HLA-A*0201 is shown as a cartoon in white. (C) Structure comparison of the B pocket between RLA-A1 and HLA-A*0201. (D) The different amino acids comprising the B pocket between RLA-A1 (cyan) and HLA-A*0201 (orange) are shown as sticks. (E) The B pocket of RLA-A1 is shown as cyan cavities. Ile24 of RLA-A1 is shown as cyan sticks. (F) The B pocket of HLA-A*0201 is shown as orange cavities. Ala24 of HLA-A*0201 is shown as orange sticks. (G and H) Vacuum electrostatic surface potential of the B pocket of RLA-A1 (G) and HLA-A*0201 (H). P2 residues of peptides and residues 63 and 66 of RLA-A1 and HLA-A*0201 are shown as sticks. The capacity of VP60-2 and its P2-Leu substitutions by small and aliphatic and aromatic amino acids in the second position for binding to RLA-A1 (I) and HLA-A*0201 (J) were evaluated by in vitro refolding. The binding motifs of the B pockets of RLA-A1 (K) and HLA-A*0201 (L) were validated by the renaturation method in vitro. RLA-A1 binds to peptides with aliphatic amino acids (Met, Leu, Ile, and Val). HLA-A*0201 binds not only to aliphatic amino acids (Met, Leu, Ile and Val) but also to hydrophilic amino acids (Ser and Thr).