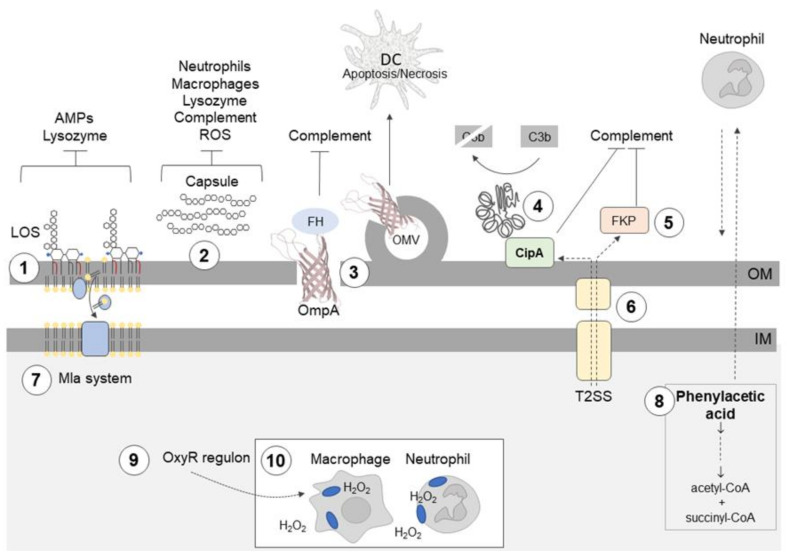
Figure 1.
Acinetobacter baumannii uses different mechanisms to evade the innate immune response. ① Hepta-acylation of lipid A in lipooligosaccharide (LOS) fortifies the outer membrane (OM) and protects A. baumannii from cationic antimicrobial peptides (AMPs), colistin, and lysozyme. ② Highly hydrophilic and negatively charged capsular polysaccharides (CPS) hinder interactions with negatively charged surfaces of neutrophils and macrophages; the capsule is also a barrier which protects against complement-mediated killing, lysozyme degradation, and reactive oxygen species (ROS). ③ Outer membrane protein A (OmpA) interacts with factor H (FH), thereby inhibiting the complement-mediated killing; OmpA induces ROS production and the death of dendritic cells (DCs). ④ CipA forms a complex with plasminogen/plasmin, which degrades the complement component C3b; CipA and ⑤ the protein killing factor (PKF) serine protease inhibit the alternative complement pathway. ⑥ The type II secretion system (T2SS) contributes to serum resistance, and it probably participates in CipA and PKF serine protease secretion. ⑦ Surface-exposed phospholipids are potential activators of the alternative complement pathway. The Mla system prevents the accumulation of phospholipids in the outer leaflet of the OM. ⑧ Phenylacetate, a derivative of phenylalanine and neutrophil attractant, is removed from the bacterial cell by conversion to acetyl-coenzyme A (CoA) and succinyl-CoA. ⑨ Enhanced catalase activity enables A. baumannii to survive in macrophages in the presence of ROS. ⑩ A. baumannii can spread during infection using neutrophils and macrophages.