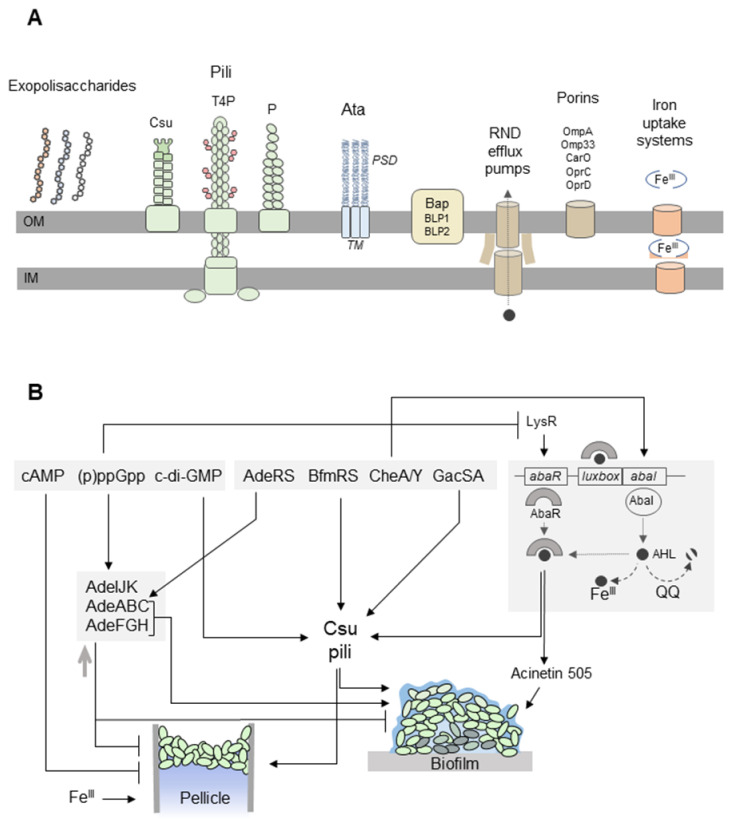
Figure 2.
A. baumannii forms biofilms on solid surfaces and pellicles at the air-liquid interface. (A) Extracellular appendages involved in biofilm/pellicle formation include exopolysaccharides (capsular polysaccharides, poly-β-(1–6)-N-acetylglucosamine (PNAG), alginate), and pili [108]. Csu pili are assembled via the chaperone-usher pathway. The CsuE adhesin, which is located at the pilus tip, exposes three hydrophobic finger-like loops that may insert into cavities in abiotic surfaces [109]. Type IV pili (T4P) are composed of PilA subunits with variable amino-acid sequences in different A. baumannii strains. Depending on the PilA structure, the pili promote twitching motility or biofilm formation [110]. The chaperone–usher P pili are overproduced in A. baumannii pellicles [105]. The homotrimeric Ata autotransporter binds to extracellular matrix components and abiotic surfaces. The transmembrane anchor domain (TM) facilitates the export of the passenger domain (PSD) to the cell surface through a pore formed by the TM. Flexible PSDs allow interactions with different surfaces [111]. Bap and Bap-like proteins (BLP1, BLP2) stabilize the three-dimensional biofilm structure on abiotic surfaces and play a role in the adhesion of A. baumannii to the host cell [112,113]. Three resistance-nodulation-division (RND) efflux pumps (AdeABC, AdeFGH, and AdeIJK) affect A. baumannii biofilm development [114,115,116]. The AdeFGH efflux pump participates in the transport of quorum-sensing (QS) molecules [116]. OmpA is responsible for the attachment of A. baumannii to plastic surfaces and epithelial cells [67]. The CarO, OprC, and OprD porins may be involved in the uptake of metabolites required for the synthesis of siderophores in pellicles [105]. The iron uptake systems, including acinetobactin and enterobactin receptors, are upregulated during pellicle formation [105,117]. (B) The formation of the A. baumannii biofilm and pellicle is regulated by the nucleotide second messengers, two-component signal transduction systems, and QS. cAMP inhibits pellicle formation [104]. The synthesis of Csu pili depends on cyclic di-GMP (c-di-GMP) [118] and the BfmRS and GacSA systems [48,119,120]. The hybrid two-component regulator CheA/Y controls the expression of Csu pili and acinetin-505 via QS [106,121]. The QS system of A. baumannii consists of an AbaI inducer and its cognate receptor AbaR. AbaI is an autoinducer synthase producing N-acyl homoserine lactone (AHL) molecules bound by AbaR. The AbaR–AHL complexes activate the synthesis of AbaI and the expression of QS-dependent genes, which in turn triggers the production of acinetin-505 and Csu pili [121,122]. Biofilm formation may be inhibited by quorum-quenching (QQ) enzymes, which degrade AHLs [123,124], as well as high concentrations of FeIII ions that bind AHLs [125]. On the other hand, FeIII ions are required for pellicle development [105,117]. The AdeABC (controlled by the two-component signal transduction AdeRS system) and AdeFGH efflux pumps participate in biofilm formation [114,116]. However, other studies revealed that the overproduction of efflux pumps may result in decreased biofilm/pellicle growth [115]. ppGpp regulates the expression of genes encoding the efflux pump’s components [126] and inhibits the production of AbaR and acinetin-505 [127].